Allergy Asthma Immunol Res.
2019 May;11(3):357-366. 10.4168/aair.2019.11.3.357.
Leukocyte Telomere Length Reflects Prenatal Stress Exposure, But Does Not Predict Atopic Dermatitis Development at 1 Year
- Affiliations
-
- 1Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea. kohyy@plaza.snu.ac.kr
- 2Department of Pediatrics, Asan Institute for Life Sciences, University of Ulsan College of Medicine, Seoul, Korea. sjhong@amc.seoul.kr
- 3Department of Pediatrics, Childhood Asthma Atopy Center, Environmental Health Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Pediatrics, CHA University Gangnam CHA Hospital, Seoul, Korea.
- 5Department of Pediatrics, Yonsei University Severance Children's Hospital, Seoul, Korea.
- 6Department of Pediatrics, Sungkyunkwan University Samsung Medical Center, Seoul, Korea.
- 7Department of Obstetrics and Gynecology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 8Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 9Department of Obstetrics and Gynecology, Yonsei University College of Medicine, Seoul, Korea.
- 10Department of Obstetrics and Gynecology, Gangnam CHA Medical Center, CHA University School of Medicine, Seoul, Korea.
- 11Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2442047
- DOI: http://doi.org/10.4168/aair.2019.11.3.357
Abstract
- PURPOSE
Prenatal maternal stress affects offspring's atopic dermatitis (AD) development, which is thought to be mediated by the oxidative stress. We aimed to evaluate the difference in leukocyte telomere length (LTL), a marker for exposure to oxidative stress, according to the prenatal stress exposure and the later AD development.
METHODS
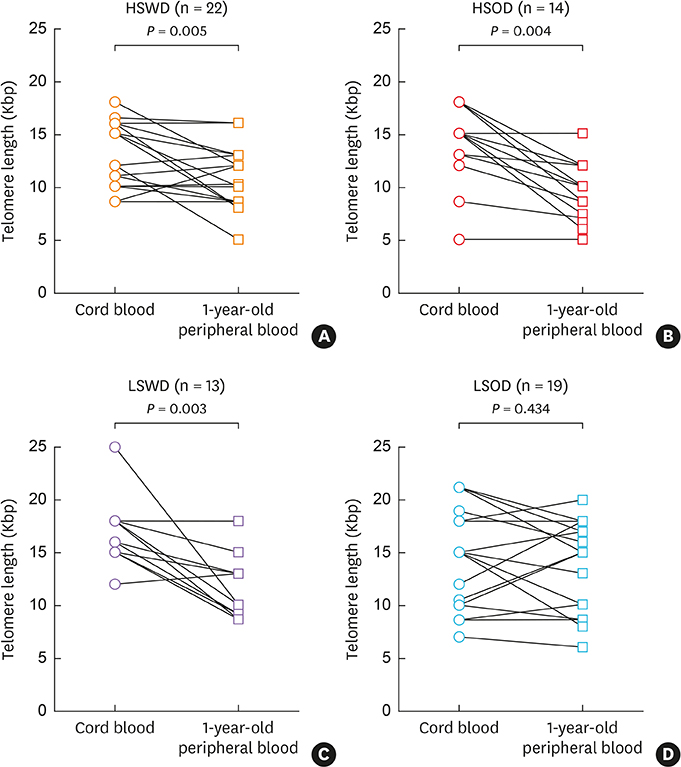
From a birth cohort (the COhort for Childhood Origin of Asthma and allergic diseases) that had displayed a good epidemiologic association between the exposure to prenatal stress and AD development in the offspring, we selected 68 pairs of samples from 4 subject groups based on the level of prenatal maternal stress and later AD development. The LTL was measured from both cord blood and 1-year peripheral blood, and their LTLs were compared between subject groups. Finally, the proportion of AD development was examined in the subject groups that are reclassified based on subjects' exposure to prenatal stress and there LTL.
RESULTS
Cord-blood LTL was shorter in prenatally stressed infants than in unstressed ones (P = 0.026), which difference was still significant when subjects became 1 year old (P = 0.008). LTL of cord blood, as well as one of the 1-year peripheral blood, was not different according to later AD development at 1 year (P = 0.915 and 0.174, respectively). Shorter LTL made no increase in the proportion of later AD development in either prenatally high-stressed or low-stressed groups (P = 1.000 and 0.473, respectively).
CONCLUSIONS
Cord-blood LTL may reflect subjects' exposure to maternal prenatal stress. However, the LTL shortening is not a risk factor of increasing AD development until the age of 1, and a longer investigation may be necessary for validation. Currently, the results doubt the role of LTL shortening as a marker for risk assessment tool for the prenatal stress associated with AD development in the offspring.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The risk of preschool asthma at 2–4 years is not associated with leukocyte telomere length at birth or at 1 year of age
Dong In Suh, Mi-Jin Kang, Yoon Mee Park, Jun-Kyu Lee, So-Yeon Lee, Youn Ho Sheen, Kyung Won Kim, Kangmo Ahn, Soo-Jong Hong
Asia Pac Allergy. 2019;9(4):. doi: 10.5415/apallergy.2019.9.e33.
Reference
-
1. Padmanabhan V, Cardoso RC, Puttabyatappa M. Developmental programming, a pathway to disease. Endocrinology. 2016; 157:1328–1340.
Article2. Smejda K, Polanska K, Merecz-Kot D, Krol A, Hanke W, Jerzynska J, et al. Maternal stress during pregnancy and allergic diseases in children during the first year of life. Respir Care. 2018; 63:70–76.
Article3. Suh DI, Chang HY, Lee E, Yang SI, Hong SJ. Prenatal maternal distress and allergic diseases in offspring: review of evidence and possible pathways. Allergy Asthma Immunol Res. 2017; 9:200–211.
Article4. Ghezzi P, Floridi L, Boraschi D, Cuadrado A, Manda G, Levic S, et al. Oxidative stress and inflammation induced by environmental and psychological stressors: a biomarker perspective. Antioxid Redox Signal. 2018; 28:852–872.
Article5. Ji H, Li XK. Oxidative stress in atopic dermatitis. Oxid Med Cell Longev. 2016; 2016:2721469.
Article6. Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017; 86:715–748.
Article7. Allsopp RC, Chang E, Kashefi-Aazam M, Rogaev EI, Piatyszek MA, Shay JW, et al. Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res. 1995; 220:194–200.8. Babizhayev MA, Savel'yeva EL, Moskvina SN, Yegorov YE. Telomere length is a biomarker of cumulative oxidative stress, biologic age, and an independent predictor of survival and therapeutic treatment requirement associated with smoking behavior. Am J Ther. 2011; 18:e209–26.
Article9. Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, et al. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology. 2013; 38:1835–1842.
Article10. Yang HJ, Lee SY, Suh DI, Shin YH, Kim BJ, Seo JH, et al. The COhort for Childhood Origin of Asthma and allergic diseases (COCOA) study: design, rationale and methods. BMC Pulm Med. 2014; 14:109.
Article11. Chang HY, Suh DI, Yang SI, Kang MJ, Lee SY, Lee E, et al. Prenatal maternal distress affects atopic dermatitis in offspring mediated by oxidative stress. J Allergy Clin Immunol. 2016; 138:468–475.e5.
Article12. Bradley KL, Bagnell AL, Brannen CL. Factorial validity of the Center for Epidemiological Studies Depression 10 in adolescents. Issues Ment Health Nurs. 2010; 31:408–412.
Article13. Hahn DW, Lee CH, Chon KK. Korean adaptation of Spielberger's STAI (K-STAI). Korean J Health Psychol. 1996; 1:1–14.14. Kim KH. Overview of atopic dermatitis. Asia Pac Allergy. 2013; 3:79–87.
Article15. Kimura M, Stone RC, Hunt SC, Skurnick J, Lu X, Cao X, et al. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat Protoc. 2010; 5:1596–1607.
Article16. Okuda K, Bardeguez A, Gardner JP, Rodriguez P, Ganesh V, Kimura M, et al. Telomere length in the newborn. Pediatr Res. 2002; 52:377–381.
Article17. Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001; 29:1165–1188.
Article18. Marchetto NM, Glynn RA, Ferry ML, Ostojic M, Wolff SM, Yao R, et al. Prenatal stress and newborn telomere length. Am J Obstet Gynecol. 2016; 215:94.e1–94.e8.
Article19. Hansen ME, Hunt SC, Stone RC, Horvath K, Herbig U, Ranciaro A, et al. Shorter telomere length in Europeans than in Africans due to polygenetic adaptation. Hum Mol Genet. 2016; 25:2324–2330.
Article20. Elbers CC, Garcia ME, Kimura M, Cummings SR, Nalls MA, Newman AB, et al. Comparison between southern blots and qPCR analysis of leukocyte telomere length in the health ABC study. J Gerontol A Biol Sci Med Sci. 2014; 69:527–531.
Article21. Send TS, Gilles M, Codd V, Wolf I, Bardtke S, Streit F, et al. Telomere length in newborns is related to maternal stress during pregnancy. Neuropsychopharmacology. 2017; 42:2407–2413.
Article22. Werlang IC, Hahn MC, Bernardi JR, Nast M, Goldani MZ, Michalowski MB. Exposure to different intrauterine environments: implications for telomere attrition in early life. J Matern Fetal Neonatal Med. 2018; 31:1–10.
Article23. Zheng T, Yu J, Oh MH, Zhu Z. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011; 3:67–73.
Article24. Loo EX, Shek LP, Goh A, Teoh OH, Chan YH, Soh SE, et al. Atopic dermatitis in early life: evidence for at least three phenotypes? results from the GUSTO study. Int Arch Allergy Immunol. 2015; 166:273–279.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The risk of preschool asthma at 2–4 years is not associated with leukocyte telomere length at birth or at 1 year of age
- How Does Obesity and Physical Activity Affect Aging?: Focused on Telomere as a Biomarker of Aging
- Measurement of Atopic Dermatitis Disability
- Associations of Perceived Stress Level, Serum Cortisol Level, and Telomere Length of Community-dwelling Adults in Korea
- Allergic March: Progression from Atopic Dermatitis to Asthma