Ann Dermatol.
2019 Apr;31(2):164-174. 10.5021/ad.2019.31.2.164.
Synthesized Ceramide Induces Growth of Dermal Papilla Cells with Potential Contribution to Hair Growth
- Affiliations
-
- 1Department of Dermatology, St. Paul's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. johnkang@catholic.ac.kr
- KMID: 2439062
- DOI: http://doi.org/10.5021/ad.2019.31.2.164
Abstract
- BACKGROUND
The ceramide is known to play an important role in the formation of intracellular lipids, and play a crucial role as a barrier for skin and hair cuticle. Recent study has revealed that ceramide has potential effect on hair growth in a mouse model. However, the role of ceramide in human dermal papilla cells (hDPCs) known to play an important role in hair growth is not well understood yet.
OBJECTIVE
The goal of this study was to investigate the effect of synthetic ceramides (oleyl and stearyl ceramides) on hair growth using hDPCs.
METHODS
hDPCs were treated with synthesized ceramides. hDPCs viability was evaluated by MTT assay. The expression of hair growth related factors were investigated by western blot, real-time polymerase chain reaction and growth factor array. The expression of β-catenin was confirmed by immunofluorescence.
RESULTS
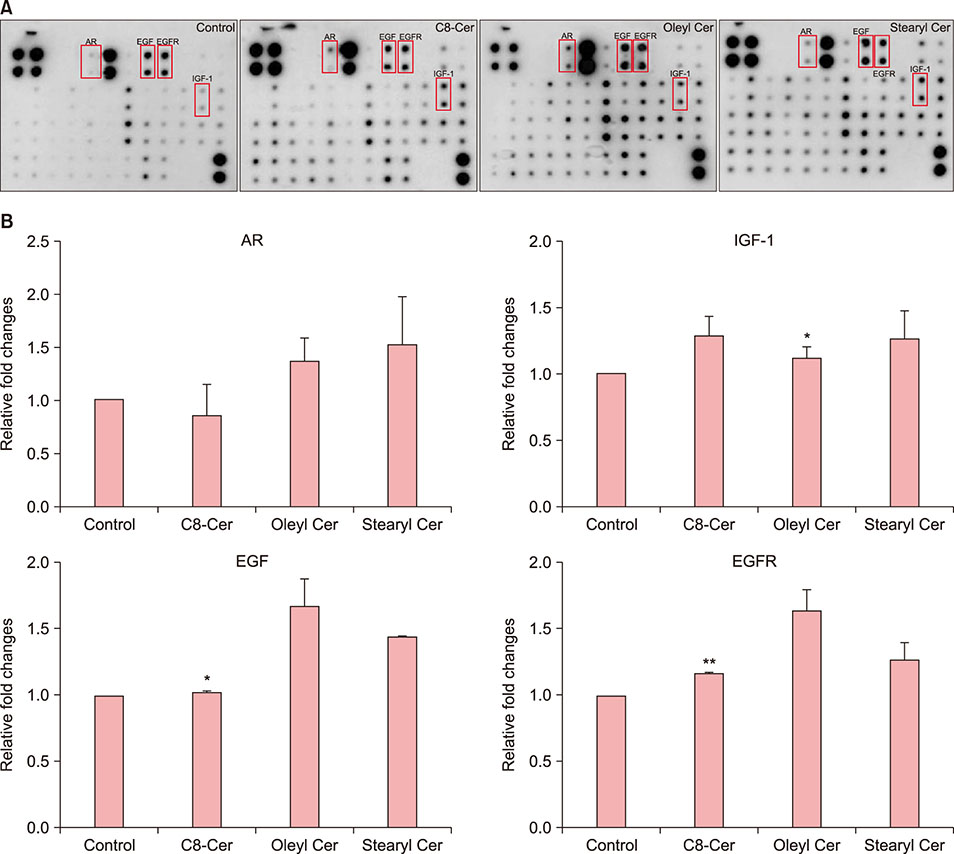
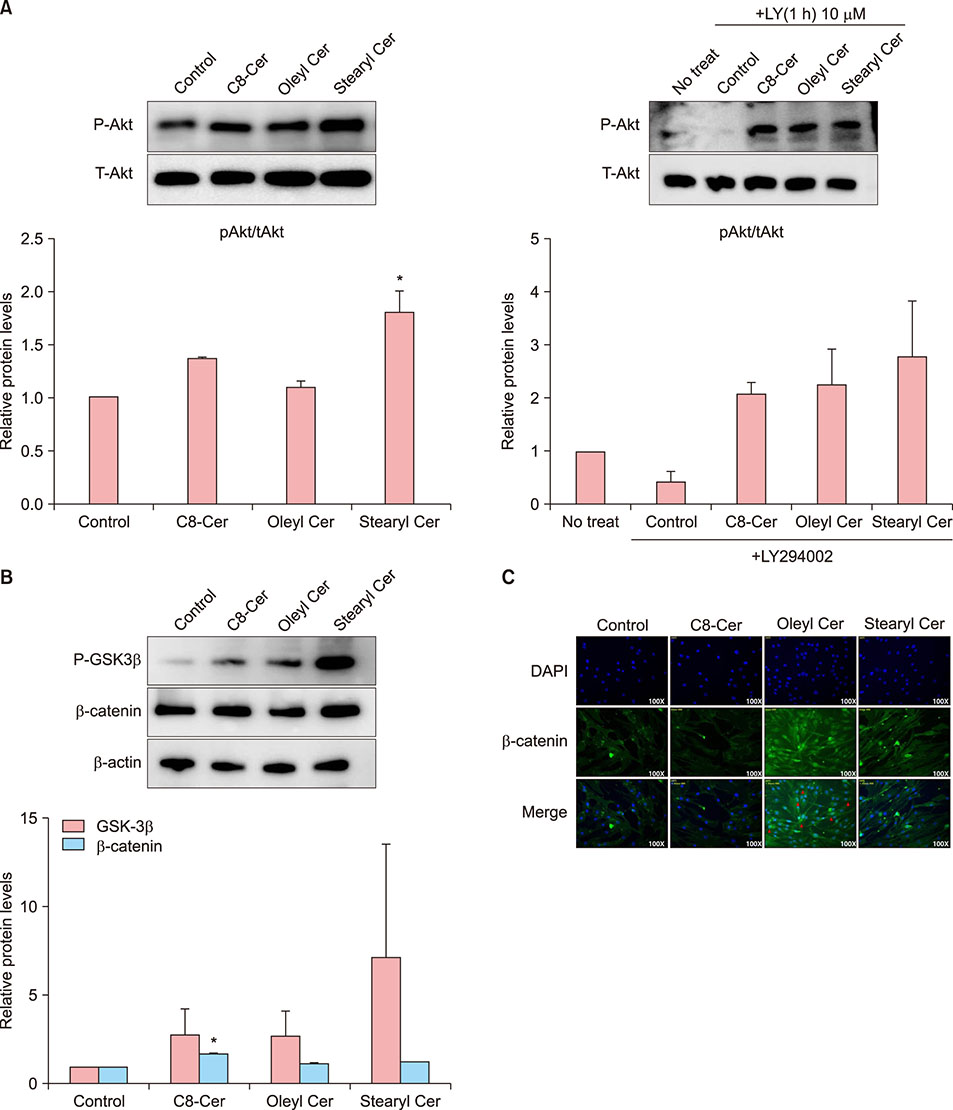
Treatment with ceramides increased the expression of proteins affecting cell proliferation such as Bcl-2, BAX, phosphorylated-ERK and Cyclin D1. Also, ceramides treatment were increased the expression of several growth factors, including epidermal growth factor family, and promote the expression of Wnt/β-catenin and BMP2/4 signaling.
CONCLUSION
Our data suggest that synthetic ceramides stimulates hair growth by induction proliferation of hDPCs via modulation of Wnt/β-catenin and BMP2/4 signaling.
Keyword
MeSH Terms
-
Animals
Blotting, Western
Cell Proliferation
Ceramides
Cyclin D1
Epidermal Growth Factor
Fluorescent Antibody Technique
Hair*
Humans
Intercellular Signaling Peptides and Proteins
Mice
Real-Time Polymerase Chain Reaction
Skin
Wnt Signaling Pathway
Ceramides
Cyclin D1
Epidermal Growth Factor
Intercellular Signaling Peptides and Proteins
Figure
Reference
-
1. Trüeb RM. Oxidative stress in ageing of hair. Int J Trichology. 2009; 1:6–14.
Article2. Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002; 2:643–653.
Article3. Takahashi T, Mamada A, Breakspear S, Itou T, Tanji N. Age-dependent changes in damage processes of hair cuticle. J Cosmet Dermatol. 2015; 14:2–8.
Article4. Lee WS, Oh TH, Chun SH, Jeon SY, Lee EY, Lee S, et al. Integral lipid in human hair follicle. J Investig Dermatol Symp Proc. 2005; 10:234–237.
Article5. Sasaki T, Hazeki K, Hazeki O, Ui M, Katada T. Permissive effect of ceramide on growth factor-induced cell proliferation. Biochem J. 1995; 311:829–834.
Article6. Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM. Role of ceramide in cellular senescence. J Biol Chem. 1995; 270:30701–30708.
Article7. Park BM, Bak SS, Shin KO, Kim M, Kim D, Jung SH, et al. Promotion of hair growth by newly synthesized ceramide mimetic compound. Biochem Biophys Res Commun. 2017; 491:173–177.
Article8. Peters F, Vorhagen S, Brodesser S, Jakobshagen K, Brüning JC, Niessen CM, et al. Ceramide synthase 4 regulates stem cell homeostasis and hair follicle cycling. J Invest Dermatol. 2015; 135:1501–1509.
Article9. Jeong KH, Joo HJ, Kim JE, Park YM, Kang H. Effect of mycophenolic acid on proliferation of dermal papilla cells and induction of anagen hair follicles. Clin Exp Dermatol. 2015; 40:894–902.
Article10. Li W, Man XY, Li CM, Chen JQ, Zhou J, Cai SQ, et al. VEGF induces proliferation of human hair follicle dermal papilla cells through VEGFR-2-mediated activation of ERK. Exp Cell Res. 2012; 318:1633–1640.
Article11. Han JH, Kwon OS, Chung JH, Cho KH, Eun HC, Kim KH. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J Dermatol Sci. 2004; 34:91–98.
Article12. Rishikaysh P, Dev K, Diaz D, Qureshi WM, Filip S, Mokry J. Signaling involved in hair follicle morphogenesis and development. Int J Mol Sci. 2014; 15:1647–1670.
Article13. Zhang J, He XC, Tong WG, Johnson T, Wiedemann LM, Mishina Y, et al. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells. 2006; 24:2826–2839.
Article14. Uchida Y. Ceramide signaling in mammalian epidermis. Biochim Biophys Acta. 2014; 1841:453–462.
Article15. Méndez S, Manich AM, Martí M, Parra JL, Coderch L. Damaged hair retrieval with ceramide-rich liposomes. J Cosmet Sci. 2011; 62:565–577.16. Kwack MH, Kang BM, Kim MK, Kim JC, Sung YK. Minoxidil activates β-catenin pathway in human dermal papilla cells: a possible explanation for its anagen prolongation effect. J Dermatol Sci. 2011; 62:154–159.
Article17. Elliott K, Stephenson TJ, Messenger AG. Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: implications for the control of hair follicle size and androgen responses. J Invest Dermatol. 1999; 113:873–877.
Article18. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007; 35:495–516.
Article19. Lutter M, Perkins GA, Wang X. The pro-apoptotic Bcl-2 family member tBid localizes to mitochondrial contact sites. BMC Cell Biol. 2001; 2:22.20. Hanada M, Aimé-Sempé C, Sato T, Reed JC. Structurefunction analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J Biol Chem. 1995; 270:11962–11969.21. Stenn KS, Lawrence L, Veis D, Korsmeyer S, Seiberg M. Expression of the bcl-2 protooncogene in the cycling adult mouse hair follicle. J Invest Dermatol. 1994; 103:107–111.
Article22. Mathias S, Peña LA, Kolesnick RN. Signal transduction of stress via ceramide. Biochem J. 1998; 335:465–480.
Article23. Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002; 12:9–18.
Article24. Joo HJ, Jeong KH, Kim JE, Kang H. Various wavelengths of light-emitting diode light regulate the proliferation of human dermal papilla cells and hair follicles via Wnt/β-catenin and the extracellular signal-regulated kinase pathways. Ann Dermatol. 2017; 29:747–754.
Article25. Yang K, Hitomi M, Stacey DW. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Div. 2006; 1:32.
Article26. Ahn SY, Pi LQ, Hwang ST, Lee WS. Effect of IGF-I on hair growth is related to the anti-apoptotic effect of IGF-I and up-regulation of PDGF-A and PDGF-B. Ann Dermatol. 2012; 24:26–31.
Article27. Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009; 8:627–644.
Article28. Monick MM, Carter AB, Robeff PK, Flaherty DM, Peterson MW, Hunninghake GW. Lipopolysaccharide activates Akt in human alveolar macrophages resulting in nuclear accumulation and transcriptional activity of beta-catenin. J Immunol. 2001; 166:4713–4720.
Article29. Kandyba E, Leung Y, Chen YB, Widelitz R, Chuong CM, Kobielak K. Competitive balance of intrabulge BMP/Wnt signaling reveals a robust gene network ruling stem cell homeostasis and cyclic activation. Proc Natl Acad Sci U S A. 2013; 110:1351–1356.
Article30. DasGupta R, Kaykas A, Moon RT, Perrimon N. Functional genomic analysis of the Wnt-wingless signaling pathway. Science. 2005; 308:826–833.
Article31. Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000; 14:1181–1185.
Article32. Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001; 107:69–82.
Article33. Ouji Y, Yoshikawa M, Shiroi A, Ishizaka S. Promotion of hair follicle development and trichogenesis by Wnt-10b in cultured embryonic skin and in reconstituted skin. Biochem Biophys Res Commun. 2006; 345:581–587.
Article34. Li YH, Zhang K, Ye JX, Lian XH, Yang T. Wnt10b promotes growth of hair follicles via a canonical Wnt signalling pathway. Clin Exp Dermatol. 2011; 36:534–540.
Article35. Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008; 451:340–344.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Vaseular Endothelial Growth Factors on Hair Growth in Vitro
- Effects of Alopecia Areata Serum on Proliferation of Cultured Dermal Papilla Cells
- Effect of Chrysanthemum zawadskii Extract on Dermal Papilla Cell Proliferation and Hair Growth
- Effects of Substance P on the Expression of Various Factors to control Hair Growth in Human Hair Follicle Culture
- Effects of Calcitonin Gene-Related Peptide on the Hair Growth in Human Hair Follicle Organ Culture