Cancer Res Treat.
2019 Jan;51(1):267-279. 10.4143/crt.2018.085.
Gastric Mucosal Atrophy Impedes Housekeeping Gene Methylation in Gastric Cancer Patients
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Department of Microbiology, College of Medicine, The Catholic University of Korea, Seoul, Korea. hongsjin@catholic.ac.kr
- 3Department of Preventive Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 4Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2437618
- DOI: http://doi.org/10.4143/crt.2018.085
Abstract
- PURPOSE
Helicobacter pylori infection induces phenotype-stabilizing methylation and promotes gastric mucosal atrophy that can inhibit CpG-island methylation. Relationship between the progression of gastric mucosal atrophy and the initiation of CpG-island methylation was analyzed to delineate epigenetic period for neoplastic transformation.
MATERIALS AND METHODS
Normal-appearing gastric mucosa was biopsied from 110 H. pylori-positive controls, 95 H. pylori-negative controls, 99 gastric cancer patients, and 118 gastric dysplasia patients. Gastric atrophy was assessed using endoscopic-atrophic-border score. Methylation-variable sites of eight CpG-island genes adjacent to Alu (CDH1, ARRDC4, PPARG, and TRAPPC2L) or LTR (MMP2, CDKN2A, RUNX2, and RUNX3) retroelements and stomach-specific TFF3 gene were analyzed using radioisotope-labeled methylation-specific polymerase chain reaction.
RESULTS
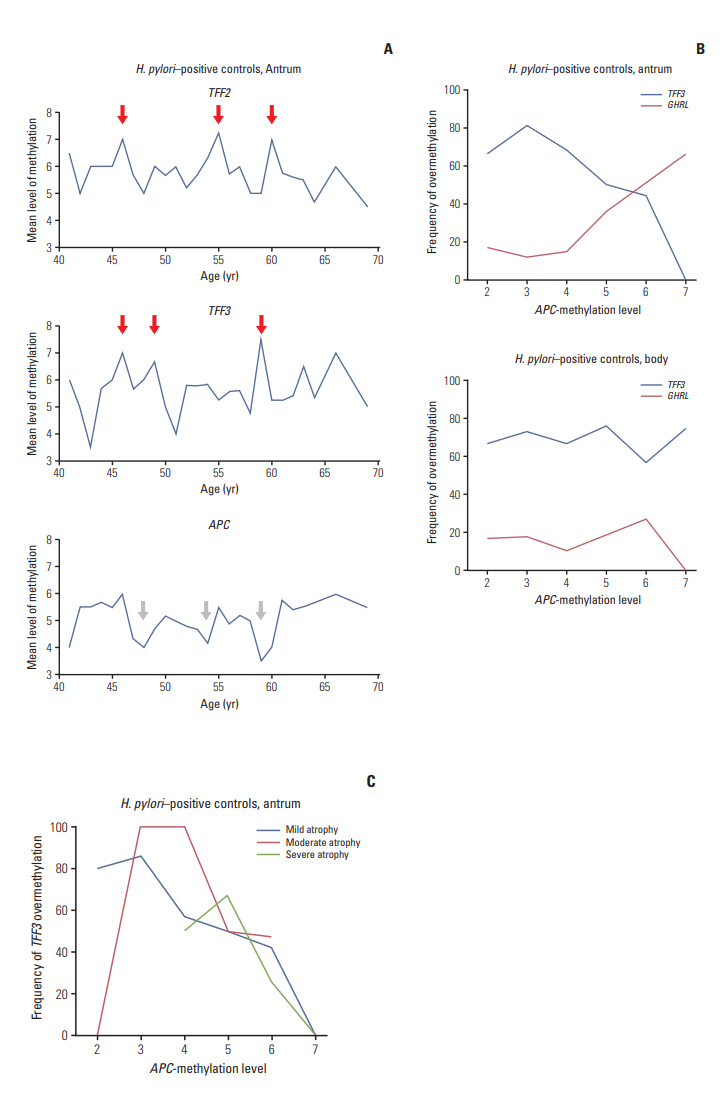
Mean ages of H. pylori-positive controls with mild, moderate, and severe atrophy were 51, 54, and 65 years and those of H. pylori-associated TFF3 overmethylation at the three atrophic levels (51, 58, and 63 years) tended to be periodic. Alu-adjacent overmethylation (50 years) was earlier than TFF3 overmethylation (58 years) in H. pylori-positive controls with moderate atrophy. Cancer patients with moderate atrophy showed late Alu-adjacent (58 years) overmethylation and frequent LTR-adjacent overmethylation. LTR-adjacent overmethylation was frequent in cancer (66 years) and dysplasia (68 years) patients with severe atrophy.
CONCLUSION
Atrophic progression is associated with gastric cancer at moderate level by impeding the initiation of Alu-adjacent methylation. LTR-adjacent methylation is increased in cancer patients and subsequently in dysplasia patients.
MeSH Terms
Figure
Reference
-
References
1. Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998; 114:1169–79.
Article2. Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007; 133:659–72.
Article3. Yoshida T, Kato J, Inoue I, Yoshimura N, Deguchi H, Mukoubayashi C, et al. Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int J Cancer. 2014; 134:1445–57.4. Kim H, Hwang Y, Sung H, Jang J, Ahn C, Kim SG, et al. Effectiveness of gastric cancer screening on gastric cancer incidence and mortality in a community-based prospective cohort. Cancer Res Treat. 2018; 50:582–9.
Article5. Whiting JL, Sigurdsson A, Rowlands DC, Hallissey MT, Fielding JW. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut. 2002; 50:378–81.
Article6. Lee JH, Abraham SC, Kim HS, Nam JH, Choi C, Lee MC, et al. Inverse relationship between APC gene mutation in gastric adenomas and development of adenocarcinoma. Am J Pathol. 2002; 161:611–8.
Article7. Shibata D. Inferring human stem cell behaviour from epigenetic drift. J Pathol. 2009; 217:199–205.
Article8. Korbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, et al. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med. 2002; 346:738–46.
Article9. Yu S, Yang M, Nam KT. Mouse models of gastric carcinogenesis. J Gastric Cancer. 2014; 14:67–86.
Article10. Wang F, Meng W, Wang B, Qiao L. Helicobacter pyloriinduced gastric inflammation and gastric cancer. Cancer Lett. 2014; 345:196–202.
Article11. Rhyu MG, Oh JH, Hong SJ. Species-specific role of gene-adjacent retroelements in human and mouse gastric carcinogenesis. Int J Cancer. 2018; 142:1520–7.
Article12. Moriguchi S, Kamakura T, Odaka T, Nose Y, Maehara Y, Korenaga D, et al. Clinical features of the differentiated and undifferentiated types of advanced gastric carcinoma: univariate and multivariate analyses. J Surg Oncol. 1991; 48:202–6.
Article13. Tatematsu M, Tsukamoto T, Inada K. Stem cells and gastric cancer: role of gastric and intestinal mixed intestinal metaplasia. Cancer Sci. 2003; 94:135–41.
Article14. Hong SJ, Lee HJ, Oh JH, Jung SH, Min KO, Choi SW, et al. Age-related methylation patterning of housekeeping genes and tissue-specific genes is distinct between the stomach antrum and body. Epigenomics. 2013; 5:283–99.
Article15. Oh JH, Rhyu MG, Jung SH, Choi SW, Kim SI, Hong SJ. Slow overmethylation of housekeeping genes in the body mucosa is associated with the risk for gastric cancer. Cancer Prev Res (Phila). 2014; 7:585–95.
Article16. Ji H, Ehrlich LI, Seita J, Murakami P, Doi A, Lindau P, et al. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature. 2010; 467:338–42.
Article17. Rhyu MG, Oh JH, Hong SJ. Epigenetic implication of geneadjacent retroelements in Helicobacter pylori-infected adults. Epigenomics. 2012; 4:527–35.
Article18. Maekita T, Nakazawa K, Mihara M, Nakajima T, Yanaoka K, Iguchi M, et al. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006; 12(3 Pt 1):989–95.
Article19. Kim YH, Hong SJ, Jung YC, Kim SJ, Seo EJ, Choi SW, et al. The 5'-end transitional CpGs between the CpG islands and retroelements are hypomethylated in association with loss of heterozygosity in gastric cancers. BMC Cancer. 2006; 6:180.
Article20. Kang MI, Kim HS, Jung YC, Kim YH, Hong SJ, Kim MK, et al. Transitional CpG methylation between promoters and retroelements of tissue-specific genes during human mesenchymal cell differentiation. J Cell Biochem. 2007; 102:224–39.
Article21. Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969; 1:87–97.
Article22. Hong SJ, Kang MI, Oh JH, Jung YC, Kim YH, Kim SJ, et al. DNA methylation and expression patterns of key tissue-specific genes in adult stem cells and stomach tissues. J Korean Med Sci. 2009; 24:918–29.
Article23. Weber M, Hellmann I, Stadler MB, Ramos L, Paabo S, Rebhan M, et al. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet. 2007; 39:457–66.
Article24. Polo JM, Anderssen E, Walsh RM, Schwarz BA, Nefzger CM, Lim SM, et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell. 2012; 151:1617–32.
Article25. Yoshida T, Kato J, Maekita T, Yamashita S, Enomoto S, Ando T, et al. Altered mucosal DNA methylation in parallel with highly active Helicobacter pylori-related gastritis. Gastric Cancer. 2013; 16:488–97.
Article26. Liu Y, Uemura N, Xiao SD, Tytgat GN, Kate FJ. Agreement between endoscopic and histological gastric atrophy scores. J Gastroenterol. 2005; 40:123–7.
Article27. Inoue M, Tajima K, Matsuura A, Suzuki T, Nakamura T, Ohashi K, et al. Severity of chronic atrophic gastritis and subsequent gastric cancer occurrence: a 10-year prospective cohort study in Japan. Cancer Lett. 2000; 161:105–12.
Article28. Sapari NS, Loh M, Vaithilingam A, Soong R. Clinical potential of DNA methylation in gastric cancer: a meta-analysis. PLoS One. 2012; 7:e36275.
Article29. Meining A, Morgner A, Miehlke S, Bayerdorffer E, Stolte M. Atrophy-metaplasia-dysplasia-carcinoma sequence in the stomach: a reality or merely an hypothesis? Best Pract Res Clin Gastroenterol. 2001; 15:983–98.
Article30. de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008; 134:945–52.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Periodic Methylation Patterns in the Background Mucosa of Gastric Cancer
- DNA Methylation as Surrogate Marker For Gastric Cancer
- Methylation of P16 and hMLH1 in Gastric Carcinoma
- Gastrokine 1 Expression in the Human Gastric Mucosa Is Closely Associated with the Degree of Gastritis and DNA Methylation
- Effectiveness of Helicobacter pylori Eradication before Endoscopic Resection