Cancer Res Treat.
2019 Jan;51(1):80-89. 10.4143/crt.2017.500.
Expression of Immunoproteasome Subunit LMP7 in Breast Cancer and Its Association with Immune-Related Markers
- Affiliations
-
- 1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. backlila@gmail.com
- 2Asan Center for Cancer Genome Discovery, Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2437601
- DOI: http://doi.org/10.4143/crt.2017.500
Abstract
- PURPOSE
In the presence of interferon, proteasome subunits are replaced by their inducible counterparts to form an immunoproteasome (IP) plays a key role in generation of antigenic peptides presented by MHC class I molecules, leading to elicitation of a T cell"’mediated immune response. Although the roles of IP in other cancers, and inflammatory diseases have been extensively studied, its significance in breast cancer is unclear.
MATERIALS AND METHODS
We investigated the expression of LMP7, an IP subunit, and its relationship with immune system components in two breast cancer cohorts.
RESULTS
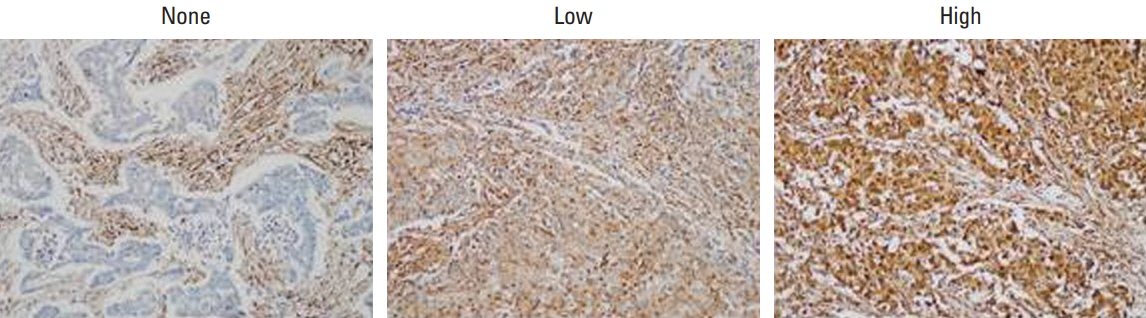
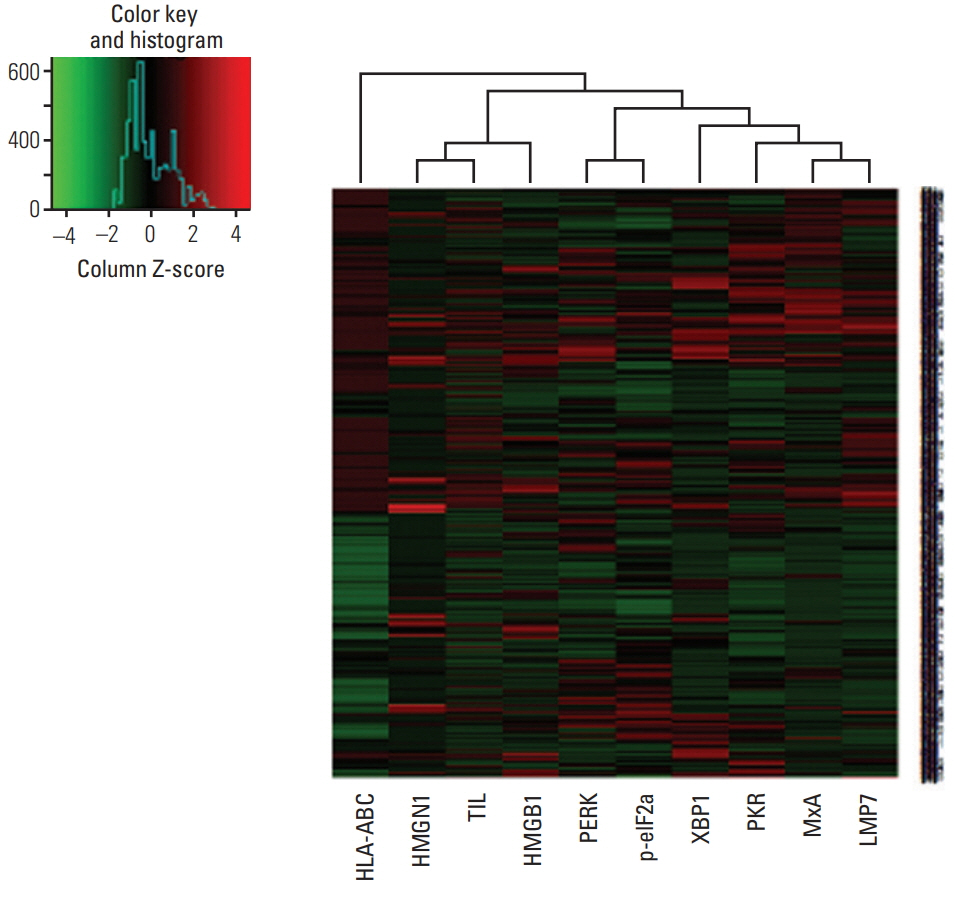
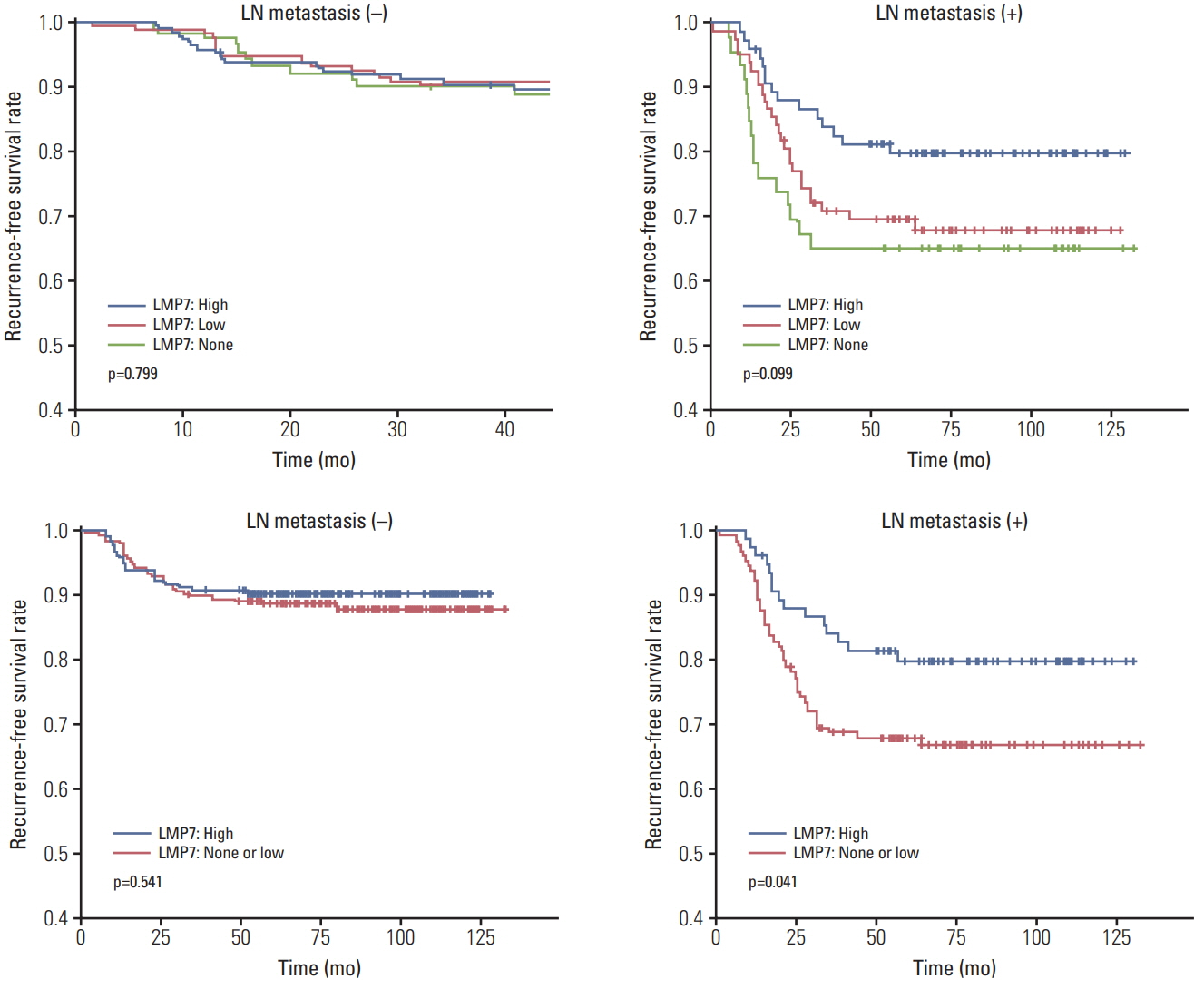
In 668 consecutive breast cancer cohort, 40% of tumors showed high level of LMP7 expression, and tumors with high expression of LMP7 had more tumor-infiltrating lymphocytes (TILs) in each subtype of breast cancer. In another cohort of 681 triple-negative breast cancer patients cohort, the expression of LMP7 in tumor cells was significantly correlated with the amount of TILs and the expression of interferon-associated molecules (MxA [p < 0.001] and PKR [p < 0.001]), endoplasmic reticulum stress-associated molecules (PERK [p=0.012], p-eIF2a [p=0.001], and XBP1 [p < 0.001]), and damage-associated molecular patterns (HMGN1 [p < 0.001] and HMGB1 [p < 0.001]). Patients with higher LMP7 expression had better disease-free survival outcomes than those with no or low expression in the positive lymph node metastasis group (p=0.041).
CONCLUSION
Close association between the TIL levels and LMP7 expression in breast cancer indicates that better antigen presentation through greater LMP7 expression might be associated with more TILs.
Keyword
MeSH Terms
-
Antigen Presentation
Breast Neoplasms*
Breast*
Cohort Studies
Disease-Free Survival
Endoplasmic Reticulum
HLA Antigens
HMGB1 Protein
Humans
Immune System
Interferons
Lymph Nodes
Lymphocytes, Tumor-Infiltrating
Neoplasm Metastasis
Peptides
Proteasome Endopeptidase Complex
Triple Negative Breast Neoplasms
HLA Antigens
HMGB1 Protein
Interferons
Peptides
Proteasome Endopeptidase Complex
Figure
Reference
-
References
1. van Rooijen JM, Stutvoet TS, Schroder CP, de Vries EG. Immunotherapeutic options on the horizon in breast cancer treatment. Pharmacol Ther. 2015; 156:90–101.
Article2. Kast J. Immunoproteasome deficiency in non-small cell lung cancer and its relevance to immunotherapy. J Thorac Dis. 2016; 8:E1082–6.
Article3. Shashova EE, Lyupina YV, Glushchenko SA, Slonimskaya EM, Savenkova OV, Kulikov AM, et al. Proteasome functioning in breast cancer: connection with clinical-pathological factors. PLoS One. 2014; 9:e109933.
Article4. Kaur G, Batra S. Emerging role of immunoproteasomes in pathophysiology. Immunol Cell Biol. 2016; 94:812–20.
Article5. Kim JY, Heo SH, Song IH, Park IA, Kim YA, Gong G, et al. Activation of the PERK-eIF2alpha pathway is associated with tumor-infiltrating lymphocytes in HER2-positive breast cancer. Anticancer Res. 2016; 36:2705–11.6. Lee HJ, Park IA, Song IH, Shin SJ, Kim JY, Yu JH, et al. Tertiary lymphoid structures: prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer. J Clin Pathol. 2016; 69:422–30.
Article7. Lee HJ, Song IH, Park IA, Heo SH, Kim YA, Ahn JH, et al. Differential expression of major histocompatibility complex class I in subtypes of breast cancer is associated with estrogen receptor and interferon signaling. Oncotarget. 2016; 7:30119–32.
Article8. Park IA, Heo SH, Song IH, Kim YA, Park HS, Bang WS, et al. Endoplasmic reticulum stress induces secretion of high-mobility group proteins and is associated with tumor-infiltrating lymphocytes in triple-negative breast cancer. Oncotarget. 2016; 7:59957–64.
Article9. Kim YA, Lee HJ, Heo SH, Park HS, Park SY, Bang W, et al. MxA expression is associated with tumor-infiltrating lymphocytes and is a prognostic factor in triple-negative breast cancer. Breast Cancer Res Treat. 2016; 156:597–606.
Article10. Lee HJ, Seo AN, Park SY, Kim JY, Park JY, Yu JH, et al. Low prognostic implication of fibroblast growth factor family activation in triple-negative breast cancer subsets. Ann Surg Oncol. 2014; 21:1561–8.
Article11. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010; 134:e48–72.12. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014; 138:241–56.
Article13. Torigoe T, Asanuma H, Nakazawa E, Tamura Y, Hirohashi Y, Yamamoto E, et al. Establishment of a monoclonal anti-pan HLA class I antibody suitable for immunostaining of formalin-fixed tissue: unusually high frequency of down-regulation in breast cancer tissues. Pathol Int. 2012; 62:303–8.
Article14. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012; 490:61–70.15. Cabrera CM, Jimenez P, Cabrera T, Esparza C, Ruiz-Cabello F, Garrido F. Total loss of MHC class I in colorectal tumors can be explained by two molecular pathways: beta2-microglobulin inactivation in MSI-positive tumors and LMP7/TAP2 downregulation in MSI-negative tumors. Tissue Antigens. 2003; 61:211–9.16. Miller Z, Ao L, Kim KB, Lee W. Inhibitors of the immunoproteasome: current status and future directions. Curr Pharm Des. 2013; 19:4140–51.
Article17. Rouette A, Trofimov A, Haberl D, Boucher G, Lavallee VP, D'Angelo G, et al. Expression of immunoproteasome genes is regulated by cell-intrinsic and -extrinsic factors in human cancers. Sci Rep. 2016; 6:34019.
Article18. Lee HJ, Seo JY, Ahn JH, Ahn SH, Gong G. Tumor-associated lymphocytes predict response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2013; 16:32–9.
Article19. Lee SJ, Hwang CS, Kim YK, Lee HJ, Ahn SJ, Shin N, et al. Expression of myxovirus resistance A (MxA) is associated with tumor-infiltrating lymphocytes in human epidermal growth factor receptor 2 (HER2)-positive breast cancers. Cancer Res Treat. 2017; 49:313–21.
Article20. Ma W, Vigneron N, Chapiro J, Stroobant V, Germeau C, Boon T, et al. A MAGE-C2 antigenic peptide processed by the immunoproteasome is recognized by cytolytic T cells isolated from a melanoma patient after successful immunotherapy. Int J Cancer. 2011; 129:2427–34.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression Pattern of Immunoproteasome Subunits in Human Thymus
- Evaluation of ImmunoproteasomeSpecific Proteolytic Activity Using Fluorogenic Peptide Substrates
- Matrix Metallopeptidase 3 Polymorphisms: Emerging genetic Markers in Human Breast Cancer Metastasis
- Polymorphism of Antigen Processing ( TAP, HLA-DM, LMP ) Genes in Korean Population
- Immunoproteasome induction is suppressed in hepatitis C virus-infected cells in a protein kinase R-dependent manner