Anesth Pain Med.
2018 Jan;13(1):82-92. 10.17085/apm.2018.13.1.82.
Differential expression of spinal γ-aminobutyric acid and opioid receptors modulates the analgesic effects of intrathecal curcumin on postoperative/inflammatory pain in rats
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chonnam National University Medical School, Gwangju, Korea. mhyoon@chonnam.ac.kr
- 2Center for Creative Biomedical Scientists at Chonnam National University, Gwangju, Korea.
- KMID: 2436061
- DOI: http://doi.org/10.17085/apm.2018.13.1.82
Abstract
- BACKGROUND
Curcumin is traditionally used as an herbal medicine. We explored the efficacy of intrathecal curcumin in relieving both postoperative and inflammatory pain and elucidated the mechanisms of action of curcumin interacting with γ-aminobutyric acid (GABA) and opioid receptors at the spinal level.
METHODS
Experimental pain was induced in male Sprague-Dawley rats via paw incision or injection of intraplantar carrageenan. After examination of the effects of intrathecal curcumin on the pain, GABA and opioid receptor antagonists were intrathecally administered to explore the involvement of GABA or opioid receptors on the effect of curcumin. Additionally, the expression levels of the GABA and opioid receptors were assessed.
RESULTS
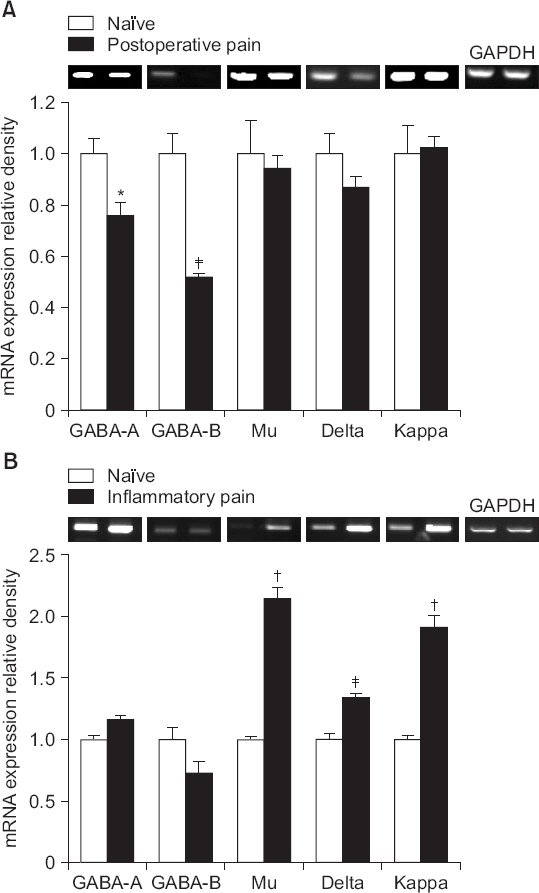
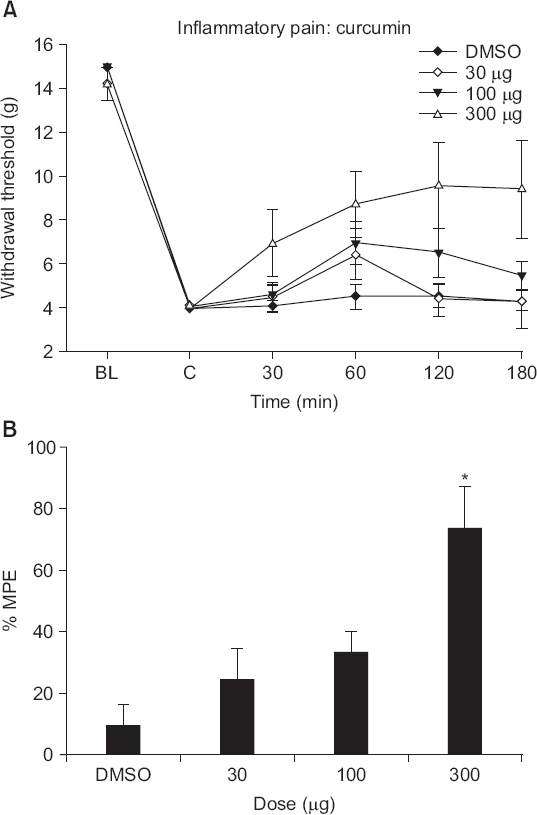
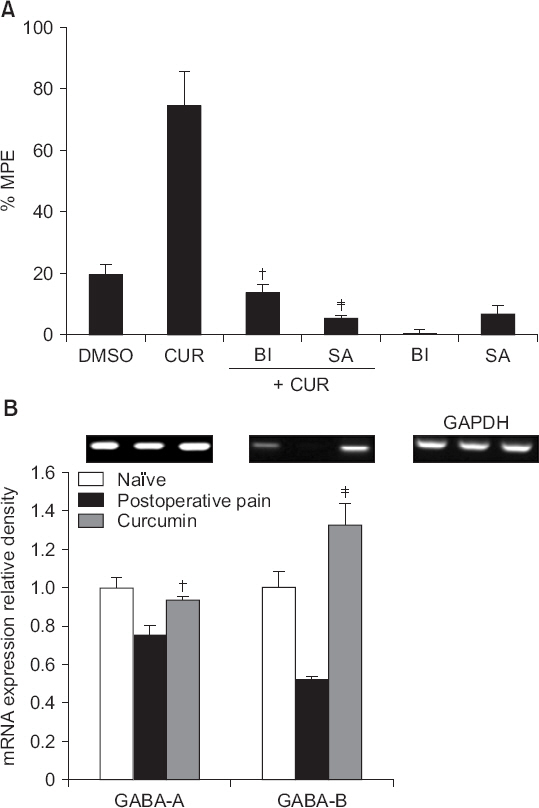
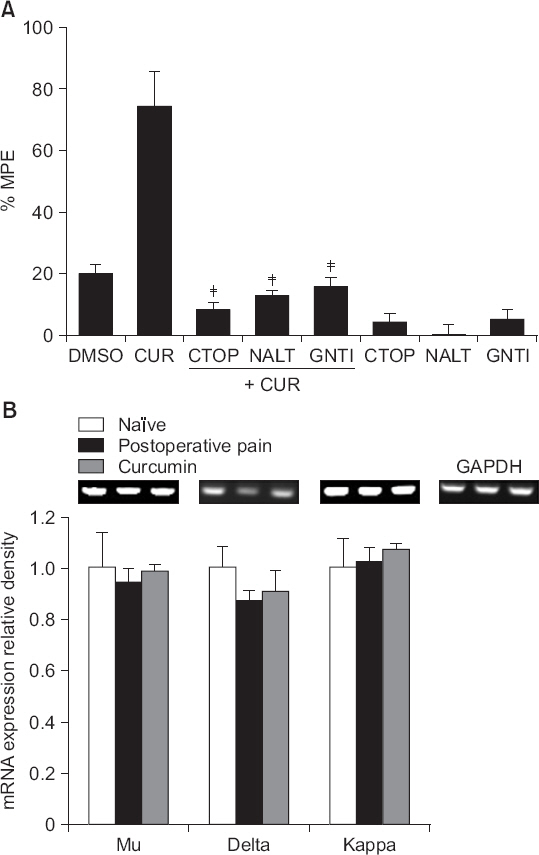
Intrathecal curcumin reduced the withdrawal threshold of both incisional surgery- and carrageenan injection-induced nociception. Intrathecal GABA and opioid receptor antagonists reversed the curcumin-mediated antinociception. Incisional surgery decreased the levels of the GABA receptors mRNA, but little changed the levels of the opioid receptors mRNA. Carrageenan injection increased the levels of the opioid receptors mRNA, but not the GABA receptors mRNA levels. Intrathecal curcumin increased or decreased the levels of GABA receptors mRNA and opioid receptors mRNA in the spinal cords of incised or carrageenan-injected rats, respectively.
CONCLUSIONS
Intrathecal curcumin was effective to postoperative and inflammatory pain and such antinociception of curcumin was antagonized by both GABA and opioid receptor antagonists. Also, intrathecal curcumin altered the levels of GABA and opioid receptors. Thus, spinal GABA and opioid receptors may, respectively, be directly or indirectly involved when curcumin alleviates postoperative and inflammatory pain.
Keyword
MeSH Terms
Figure
Reference
-
1. Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010; 16:1248–57. DOI: 10.1038/nm.2235. PMID: 20948530. PMCID: PMC5022111.2. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011; 152(3 Suppl):S2–15. DOI: 10.1016/j.pain.2010.09.030. PMID: 20961685. PMCID: PMC3268359.3. Fishman SM, Ballantyne JE, Rathmell JP, Bonica JJ. Bonica's management of pain. 4th ed. Baltimore, MD: Lippincott Williams & Wilkins;2010.4. Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa). J Altern Complement Med. 2003; 9:161–8. DOI: 10.1089/107555303321223035. PMID: 12676044.5. Chen JJ, Dai L, Zhao LX, Zhu X, Cao S, Gao YJ. Intrathecal curcumin attenuates pain hypersensitivity and decreases spinal neuroinflammation in rat model of monoarthritis. Sci Rep. 2015; 5:10278. DOI: 10.1038/srep10278. PMID: 25988362. PMCID: PMC4437313.6. Lee JH, Kim YD, Jung HC, Cheong YK. The effect of intrathecal curcumin on mechanical allodynia in rats after L5 spinal nerve ligation. Korean J Anesthesiol. 2014; 67(Suppl):S122–3. DOI: 10.4097/kjae.2014.67.S.S122. PMID: 25598884. PMCID: PMC4295958.7. Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976; 17:1031–6. DOI: 10.1016/0031-9384(76)90029-9.8. Lin H, Lee HG, Yoon MH. Antiallodynic effects of intrathecal tianeptine in a neuropathic pain rat. Anesth Pain Med. 2014; 9:93–7.9. Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996; 64:493–501. DOI: 10.1016/0304-3959(95)01441-1.10. Yang J, Bae HB, Ki HG, Oh JM, Kim WM, Lee HG, et al. Different role of spinal 5-HT(hydroxytryptamine)7 receptors and descending serotonergic modulation in inflammatory pain induced in formalin and carrageenan rat models. Br J Anaesth. 2014; 113:138–47. DOI: 10.1093/bja/aet336. PMID: 24129596.11. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63. DOI: 10.1016/0165-0270(94)90144-9.12. Castro AR, Pinto M, Lima D, Tavares I. Secondary hyperalgesia in the monoarthritic rat is mediated by GABAB and NK1 receptors of spinal dorsal horn neurons: a behavior and c-fos study. Neuroscience. 2006; 141:2087–95. DOI: 10.1016/j.neuroscience.2006.05.048. PMID: 16809001.13. Yoon MH, Kim KS, Lee HG, Kim CM, Kim WM, Choi JI, et al. Synergistic interaction between intrathecal ginsenosides and morphine on formalin-induced nociception in rats. J Pain. 2011; 12:774–81. DOI: 10.1016/j.jpain.2010.12.012. PMID: 21459679.14. Goodchild CS, Nadeson R, Cohen E. Supraspinal and spinal cord opioid receptors are responsible for antinociception following intrathecal morphine injections. Eur J Anaesthesiol. 2004; 21:179–85. DOI: 10.1097/00003643-200403000-00003. PMID: 15055889.15. Malcangio M, Bowery NG. GABA and its receptors in the spinal cord. Trends Pharmacol Sci. 1996; 17:457–62. DOI: 10.1016/S0165-6147(96)01013-9.16. Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002; 96:1161–7. DOI: 10.1097/00000542-200205000-00020. PMID: 11981157.17. Reichl S, Augustin M, Zahn PK, Pogatzki-Zahn EM. Peripheral and spinal GABAergic regulation of incisional pain in rats. Pain. 2012; 153:129–41. DOI: 10.1016/j.pain.2011.09.028. PMID: 22054599.18. Lee HG, Park SK, Yoon MH. Potentiation of morphine antiallodynic efficacy by ACPT-III, a group III metabotropic glutamate receptor agonist, in rat spinal nerve ligation-induced neuropathic pain. Pharmacol Biochem Behav. 2010; 96:108–13. DOI: 10.1016/j.pbb.2010.04.014. PMID: 20434481.19. Cabañero D, Célérier E, García-Nogales P, Mata M, Roques BP, Maldonado R, et al. The pro-nociceptive effects of remifentanil or surgical injury in mice are associated with a decrease in delta-opioid receptor mRNA levels: Prevention of the nociceptive response by on-site delivery of enkephalins. Pain. 2009; 141:88–96. DOI: 10.1016/j.pain.2008.10.011. PMID: 19058913.20. Castro-Lopes JM, Malcangio M, Pan BH, Bowery NG. Complex changes of GABAA and GABAB receptor binding in the spinal cord dorsal horn following peripheral inflammation or neurectomy. Brain Res. 1995; 679:289–97. DOI: 10.1016/0006-8993(95)00262-O.21. Obara I, Parkitna JR, Korostynski M, Makuch W, Kaminska D, Przewlocka B, et al. Local peripheral opioid effects and expression of opioid genes in the spinal cord and dorsal root ganglia in neuropathic and inflammatory pain. Pain. 2009; 141:283–91. DOI: 10.1016/j.pain.2008.12.006. PMID: 19147290.22. Cahill CM, Morinville A, Hoffert C, O’Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003; 101:199–208. DOI: 10.1016/S0304-3959(02)00333-0.23. Maekawa K, Minami M, Masuda T, Satoh M. Expression of mu- and kappa-, but not delta-, opioid receptor mRNAs is enhanced in the spinal dorsal horn of the arthritic rats. Pain. 1996; 64:365–71. DOI: 10.1016/0304-3959(95)00132-8.24. Han YK, Lee SH, Jeong HJ, Kim MS, Yoon MH, Kim WM. Analgesic effects of intrathecal curcumin in the rat formalin test. Korean J Pain. 2012; 25:1–6. DOI: 10.3344/kjp.2012.25.1.1. PMID: 22259709. PMCID: PMC3259131.25. De Paz-Campos MA, Ortiz MI, Chávez Piña AE, Zazueta-Beltrán L, Castañeda-Hernández G. Synergistic effect of the interaction between curcumin and diclofenac on the formalin test in rats. Phytomedicine. 2014; 21:1543–8. DOI: 10.1016/j.phymed.2014.06.015. PMID: 25442263.26. Nurullahoglu KE, Okudan N, Belviranli M, Oz M. The comparison of preemptive analgesic effects of curcumin and diclofenac. Bratisl Lek Listy. 2014; 115:757–60. DOI: 10.4149/BLL_2014_146.27. Zhao X, Wang C, Zhang JF, Liu L, Liu AM, Ma Q, et al. Chronic curcumin treatment normalizes depression-like behaviors in mice with mononeuropathy: involvement of supraspinal serotonergic system and GABAA receptor. Psychopharmacology (Berl). 2014; 231:2171–87. DOI: 10.1007/s00213-013-3368-2. PMID: 24297305.28. Zhi L, Dong L, Kong D, Sun B, Sun Q, Grundy D, et al. Curcumin acts via transient receptor potential vanilloid-1 receptors to inhibit gut nociception and reverses visceral hyperalgesia. Neurogastroenterol Motil. 2013; 25:e429–40. DOI: 10.1111/nmo.12145. PMID: 23638900.29. Greeshma N, Prasanth KG, Balaji B. Tetrahydrocurcumin exerts protective effect on vincristine induced neuropathy: Behavioral, biochemical, neurophysiological and histological evidence. Chem Biol Interact. 2015; 238:118–28. DOI: 10.1016/j.cbi.2015.06.025. PMID: 26102012.30. Lee JY, Shin TJ, Choi JM, Seo KS, Kim HJ, Yoon TG, et al. Antinociceptive curcuminoid, KMS4034, effects on inflammatory and neuropathic pain likely via modulating TRPV1 in mice. Br J Anaesth. 2013; 111:667–72. DOI: 10.1093/bja/aet176. PMID: 23719767.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analgesic Effects of Intrathecal Curcumin in the Rat Formalin Test
- Analgesic Effects and Complications of Very Low Dose Intrathecal Morphine in Postoperative Patients
- The Role of Opioid Receptor on the Analgesic Action of Intrathecal Sildenafil in Rats
- Effects of Intrathecal Drugs Acting on Metabotropic Glutamate Receptors and Interaction with Morphine on a Rat Incisional Pain
- Roles of Opioid Receptor Subtype in the Spinal Antinociception of Selective Cyclooxygenase 2 Inhibitor