Ann Lab Med.
2018 Sep;38(5):395-401. 10.3343/alm.2018.38.5.395.
Rejuvenating Aged Hematopoietic Stem Cells Through Improvement of Mitochondrial Function
- Affiliations
-
- 1Department of Laboratory Medicine, Chonnam National University Medical School and Chonnam National University Hwasun Hospital, Jeollanam-do, Korea. mgshin@jnu.ac.kr
- 2Department of Biomedical Engineering, University of California, CA, USA.
- 3College of Korean Medicine, Dongshin University, Naju, Korea. 98lani@gmail.com
- 4Brain Korea 21 Plus Project, Chonnam National University Medical School, Gwangju, Korea.
- 5Environmental Health Center for Childhood Leukemia and Cancer, Chonnam National University Medical School and Chonnam National University Hwasun Hospital, Jeollanam-do, Korea.
- KMID: 2434727
- DOI: http://doi.org/10.3343/alm.2018.38.5.395
Abstract
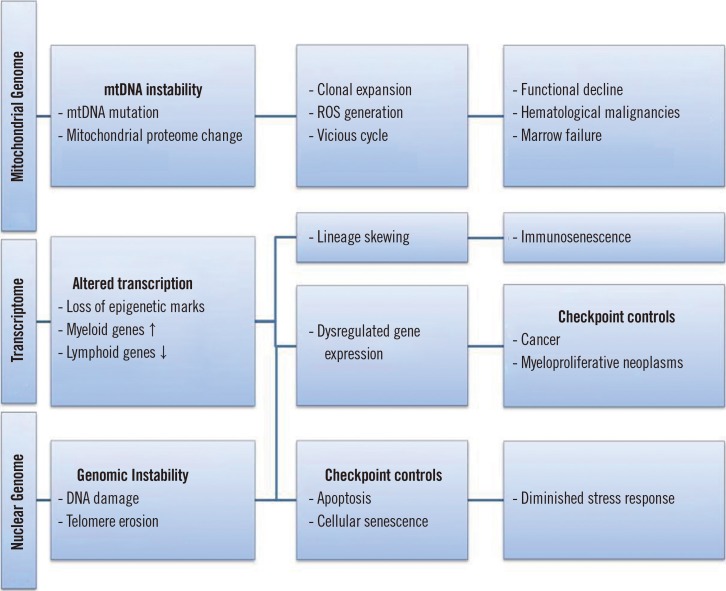
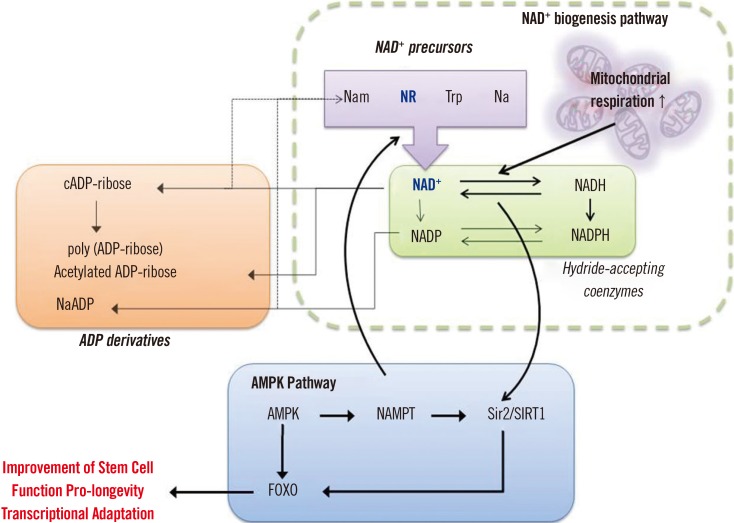
- Mitochondria are the powerhouses of the cell as well as the primary site of hematopoiesis, which also occurs in the cytoplasm. Hematopoietic stem cells (HSCs) are characterized by a very high turnover rate, and are thus considered to be relatively free from the age-related insults generated by mitochondria. However, HSCs are also subject to these age-related insults, including the incidence of myeloid proliferative diseases, marrow failure, hematopoietic neoplasms, and deterioration of the adaptive human immune system. Recently, NAD⺠dietary supplements, known as niacin or vitamin B₃, including tryptophan, nicotinic acid, nicotinamide, and the newly identified NAD⺠precursor nicotinamide riboside, have been shown to play a role in restoring adult stem cell function through the amelioration of mitochondrial dysfunction. This insight motivated a study that focused on reversing aging-related cellular dysfunction in adult mouse muscle stem cells by supplementing their diet with nicotinamide riboside. The remedial effect of nicotinamide riboside enhanced mitochondrial function in these muscle stem cells in a SIRT1-dependent manner, affecting cellular respiration, membrane potential, and production of ATP. Accordingly, numerous studies have demonstrated that sirtuins, under nuclear/mitochondrial control, have age-specific effects in determining HSC phenotypes. Based on the evidence accumulated thus far, we propose a clinical intervention for the restoration of aged HSC function by improving mitochondrial function through NAD⺠precursor supplementation.
MeSH Terms
-
Adenosine Triphosphate
Adult
Adult Stem Cells
Aging
Animals
Bone Marrow
Cell Respiration
Cytoplasm
Diet
Dietary Supplements
Hematologic Neoplasms
Hematopoiesis
Hematopoietic Stem Cells*
Humans
Immune System
Incidence
Membrane Potentials
Mice
Mitochondria
Niacin
Niacinamide
Phenotype
Sirtuins
Stem Cells
Tryptophan
Vitamins
Adenosine Triphosphate
Niacin
Niacinamide
Sirtuins
Tryptophan
Vitamins
Figure
Reference
-
1. Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005; 309:481–484. PMID: 16020738.2. Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004; 429:417–423. PMID: 15164064.3. Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013; 123:951–957. PMID: 23454757.4. Brown K, Xie S, Qiu X, Mohrin M, Shin J, Liu Y, et al. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013; 3:319–327. PMID: 23375372.5. Wang H, Diao D, Shi Z, Zhu X, Gao Y, Gao S, et al. SIRT6 controls hematopoietic stem cell homeostasis through epigenetic regulation of Wnt signaling. Cell Stem Cell. 2016; 18:495–507. PMID: 27058938.6. Mohrin M, Shin J, Liu Y, Brown K, Luo H, Xi Y, et al. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science. 2015; 347:1374–1377. PMID: 25792330.7. Rimmelé P, Bigarella CL, Liang R, Izac B, Diequez-Gonzalez R, Barbet G, et al. Aging-like phenotype and defective lineage specification in SIRT1-deleted hematopoietic stem and progenitor cells. Stem Cell Reports. 2014; 3:44–59. PMID: 25068121.8. Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016; 352:1436–1443. PMID: 27127236.9. Ryu D, Zhang H, Ropelle ER, Sorrentino V, Mázala DA, Mouchiroud L, et al. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Science Transl Med. 2016; 8:361ra139.10. Gattermann N. From sideroblastic anemia to the role of mitochondrial DNA mutations in myelodysplastic syndromes. Leuk Res. 2000; 24:141–151. PMID: 10654450.11. Unwin RD, Smith DL, Blinco D, Wilson CL, Miller CJ, Evans CA, et al. Quantitative proteomics reveals posttranslational control as a regulatory factor in primary hematopoietic stem cells. Blood. 2006; 107:4687–4694. PMID: 16507774.12. Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olsen EN, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010; 7:380–390. PMID: 20804973.13. Finkel T. Signal transduction by mitochondrial oxidants. J Biol Chem. 2012; 287:4434–4440. PMID: 21832045.14. Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007; 110:3056–3063. PMID: 17595331.15. Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004; 431:997–1002. PMID: 15496926.16. Kocabas F, Zheng J, Thet S, Copeland NG, Jenkins NA, DeBerardinis RJ, et al. Meis1 regulates the metabolic phenotype and oxidant defense of hematopoietic stem cells. Blood. 2012; 120:4963–4972. PMID: 22995899.17. Zheng J, Lu Z, Kocabas F, Böttcher RT, Costell M, Kang X, et al. Profilin 1 is essential for retention and metabolism of mouse hematopoietic stem cells in bone marrow. Blood. 2014; 123:992–1001. PMID: 24385538.18. Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Liu Y, et al. TSC–mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008; 205:2397–2408. PMID: 18809716.19. Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010; 468:653–658. PMID: 21124450.20. Norddahl GL, Pronk CJ, Wahlestedt M, Sten G, Nygren JM, Ugale A, et al. Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell Stem Cell. 2011; 8:499–510. PMID: 21549326.21. Takubo K, Nagamatsu G, Kobayashi CI, Nakamura-Ishizu A, Kobayashi H, Ikeda E, et al. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell. 2013; 12:49–61. PMID: 23290136.22. Ergen AV, Goodell MA. Mechanisms of hematopoietic stem cell aging. Exp Gerontol. 2010; 45:286–290. PMID: 20034552.23. Park CB, Larsson NG. Mitochondrial DNA mutations in disease and aging. J Cell Biol. 2011; 193:809–818. PMID: 21606204.24. Shin MG, Kajigaya S, McCoy JP Jr, Levin BC, Young NS. Marked mitochondrial DNA sequence heterogeneity in single CD34+ cell clones from normal adult bone marrow. Blood. 2004; 103:553–561. PMID: 14504082.25. Kim HR, Won SJ, Fabian C, Kang MG, Szardenings M, Shin MG. Mitochondrial DNA aberrations and pathophysiological implications in hematopoietic diseases, chronic inflammatory diseases, and cancers. Ann Lab Med. 2015; 35:1–14. PMID: 25553274.26. Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013; 155:1624–1638. PMID: 24360282.27. Lin J, Pan Y, Wang J. NAD+ and its precursors in human longevity. Quant Biol. 2015; 3:193–198.28. Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004; 117:495–502. PMID: 15137942.29. Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell. 2007; 129:473–484. PMID: 17482543.30. Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. 2007; 32:12–19. PMID: 17161604.31. van de Ven RAH, Santos D, Haigis MC. Mitochondrial sirtuins and molecular mechanisms of aging. Trends Mol Med. 2017; 23:320–331. PMID: 28285806.32. Preyat N, Leo O. Sirtuin deacylases: a molecular link between metabolism and immunity. J Leukoc Biol. 2013; 93:669–680. PMID: 23325925.33. Ramsey KM, Milis KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008; 7:78–88. PMID: 18005249.34. Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012; 15:838–847. PMID: 22682224.35. Cantó C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009; 20:325–331. PMID: 19713122.36. Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008; 28:115–130. PMID: 18429699.37. Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PloS One. 2012; 7:e42357. PMID: 22848760.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aging and impaired hematopoiesis
- Hematopoietic Stem Cell Transplantation
- Opening the era of in vivo xenotransplantation model for hematopoietic stem cell transplantation
- Current Trends and Prospect of Cell Therapy using Hematopoietic Stem Cells
- Hematopoietic Stem Cell Transplantation in Inborn Error of Metabolism