J Korean Neurosurg Soc.
2019 Jan;62(1):114-122. 10.3340/jkns.2018.0027.
Clinical Significance of Radical Surgery in the Treatment of Silent Corticotroph Adenoma
- Affiliations
-
- 1Department of Neurosurgery, Yonsei University College of Medicine, Seoul, Korea. euihyunkim@yuhs.ac
- 2Endocrine Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- 3Pituitary Tumor Center, Severance Hospital, Seoul, Korea.
- 4Department of Endocrinology, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2434353
- DOI: http://doi.org/10.3340/jkns.2018.0027
Abstract
OBJECTIVE
Silent corticotroph adenomas (SCA) are endocrine-inactive pituitary adenomas with positive immunohistochemistry staining for adrenocorticotropic hormone (ACTH). We investigated whether SCA-associated clinical profiles were more aggressive than hormonally negative adenomas (HNA).
METHODS
Among 627 patients with pathologically proven endocrine-inactive pituitary adenomas between 2004 and 2013, positive immunohistochemistry revealed 55 SCAs and 411 HNAs. Surgical outcomes and radiological and endocrinological characteristics were compared.
RESULTS
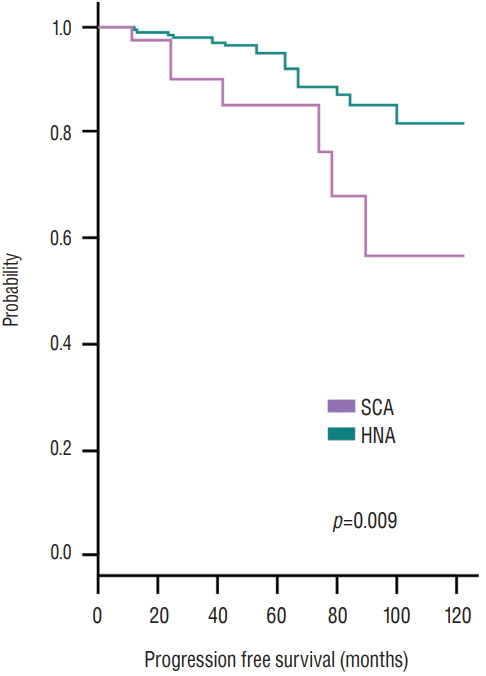
Strong female predominance was observed in the SCA group (p<0.001). Cavernous sinus invasion was identified in 22 (40%) SCA patients and 72 (17.6%) HNA patients (p<0.001). There were no differences in ACTH or cortisol levels between the two groups. The incidence of preoperative hypopituitarism and postoperative hormonal outcome did not differ between two groups. Total resection was achieved in 35 patients (63.7%) with SCA and 332 patients (80.8%) with HNA (p=0.007). When tumors were completely removed, recurrence rates were not statistically different between two groups (p=0.60). When complete resection was not achieved, tumors regrew from these remnants in seven patients (35.0%) with SCA and 12 patients (15.2%) with HNA (p=0.05).
CONCLUSION
Total surgical resection for SCA is often challenging as these tumors frequently invade a cavernous sinus. Early remnant tumor intervention is justified, because untreated residual pituitary tumors regrow when patients were followed up for a long time. Prophylactic radiotherapy is not warranted for completely resected SCAs as tumor recurrence is uncommon.
MeSH Terms
Figure
Reference
-
References
1. Alahmadi H, Lee D, Wilson JR, Hayhurst C, Mete O, Gentili F, et al. Clinical features of silent corticotroph adenomas. Acta Neurochir (Wien). 154:1493–1498. 2012.
Article2. Baldeweg SE, Pollock JR, Powell M, Ahlquist J. A spectrum of behaviour in silent corticotroph pituitary adenomas. Br J Neurosurg. 19:38–42. 2005.
Article3. Bradley KJ, Wass JAH, Turner HE. Non-functioning pituitary adenomas with positive immunoreactivity for ACTH behave more aggressively than ACTH immunonegative tumours but do not recur more frequently. Clin Endocrinol (Oxf). 58:59–64. 2003.
Article4. Cho HY, Cho SW, Kim SW, Shin CS, Park KS, Kim SY. Silent corticotroph adenomas have unique recurrence characteristics compared with other nonfunctioning pituitary adenomas. Clin Endocrinol (Oxf). 72:648–653. 2010.
Article5. Horvath E, Kovacs K, Killinger DW, Smyth HS, Platts ME, Singer W. Silent corticotropic adenomas of the human pituitary gland: a histologic, immunocytologic, and ultrastructural study. Am J Pathol. 98:617–638. 1980.6. Ioachimescu AG, Eiland L, Chhabra VS, Mastrogianakis GM, Schniederjan MJ, Brat D, et al. Silent corticotroph adenomas: Emory University cohort and comparison with ACTH-negative nonfunctioning pituitary adenomas. Neurosurgery. 71:296–303. discussion 304. 2012.7. Jahangiri A, Wagner JR, Pekmezci M, Hiniker A, Chang EF, Kunwar S, et al. A comprehensive long-term retrospective analysis of silent corticotrophic adenomas vs hormone-negative adenomas. Neurosurgery. 73:8–17. discussion 17-18. 2013.
Article8. Kim EH, Ku CR, Lee EJ, Kim SH. Extracapsular en bloc resection in pituitary adenoma surgery. Pituitary. 18:397–404. 2015.
Article9. Ku CR, Kim EH, Oh MC, Lee EJ, Kim SH. Surgical and endocrinological outcomes in the treatment of growth hormone-secreting pituitary adenomas according to the shift of surgical paradigm. Neurosurgery. 71(2 Suppl Operative):ons192-ons203; discussion ons203. 2012.
Article10. Lee EJ, Ahn JY, Noh T, Kim TS, Kim SH. Tumor tissue identification in the pseudocapsule of pituitary adenoma: should the pseudocapsule be removed for total resection of pituitary adenoma? Neurosurgery. 64:ons62-ons69; discussion ons69-ons70. 2009.
Article11. Lillehei KO, Kirschman DL, Kleinschmidt-DeMasters BK, Ridgway EC. Reassessment of the Role of Radiation Therapy in the Treatment of Endocrine-inactive Pituitary Macroadenomas. Neurosurgery. 43:432–438. discussion 438-439. 1998.
Article12. Pawlikowski M, Kunert-Radek J, Radek M. “Silent”corticotropinoma. Neuro Endocrinol Lett. 29:347–350. 2008.13. Scheithauer BW, Jaap AJ, Horvath E, Kovacs K, Lloyd RV, Meyer FB, et al. Clinically silent corticotroph tumors of the pituitary gland. Neurosurgery. 47:723–729. discussion 729-730. 2000.
Article14. Webb KM, Laurent JJ, Okonkwo DO, Lopes MB, Vance ML, Laws ER Jr. Clinical characteristics of silent corticotrophic adenomas and creation of an internet-accessible database to facilitate their multi-institutional study. Neurosurgery. 53:1076–1084. discussion 1084-1075. 2003.
Article15. Xu Z, Ellis S, Lee CC, Starke RM, Schlesinger D, Lee Vance M, et al. Silent corticotroph adenomas after stereotactic radiosurgery: a casecontrol study. Int J Radiat Oncol Biol Phys. 90:903–910. 2014.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Silent Corticotroph-cell Adenoma with Elevated Serum ACTH
- Risk factors and clinical significance of silent pulmonary embolism in patients with deep vein thrombosis
- Silent Corticotropic Adenomas of the Pituitary Gland: Cases Report
- Coincidental Aneurysms with Pituitary Adenoma: Case Report
- Effects of Oxytocin on Cell Proliferation in a Corticotroph Adenoma Cell Line