Nat Prod Sci.
2018 Dec;24(4):235-240. 10.20307/nps.2018.24.4.235.
LC/MS-based Analysis of Bioactive Compounds from the Bark of Betula platyphylla var. japonica and Their Effects on Regulation of Adipocyte and Osteoblast Differentiation
- Affiliations
-
- 1School of Pharmacy, Sungkyunkwan University, Suwon 16419, Korea. khkim83@skku.edu
- 2Sungkyun Biotech Co. Ltd., Suwon 16419, Korea.
- 3College of Korean Medicine, Gachon University, Seongnam 13120, Korea. kkang@gachon.ac.kr
- KMID: 2432417
- DOI: http://doi.org/10.20307/nps.2018.24.4.235
Abstract
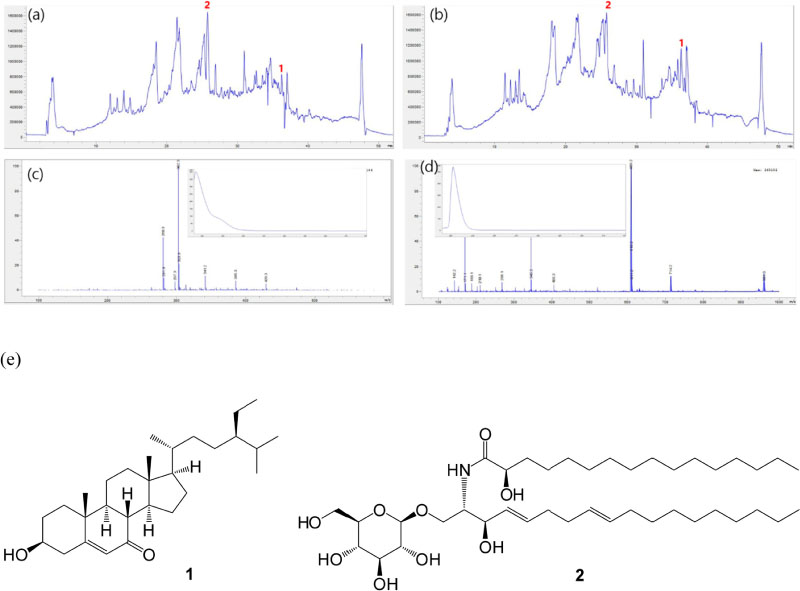
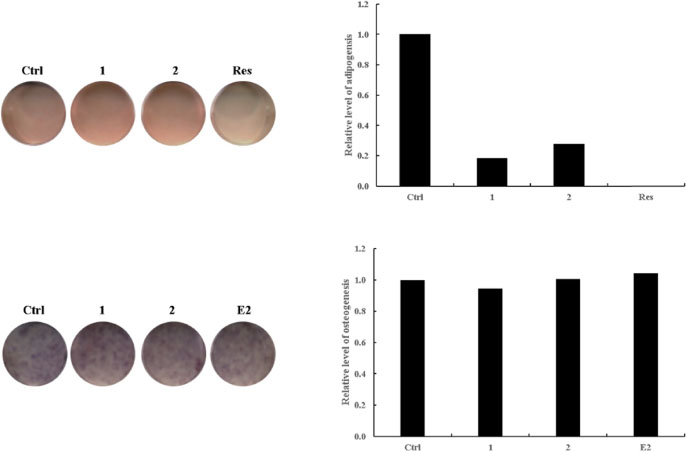
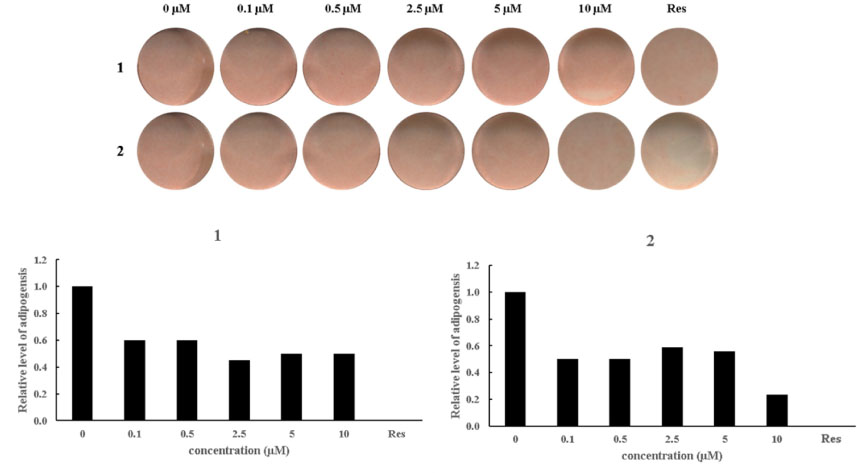
- Betula platyphylla var. japonica (Betulaceae), also known as Asian white birch, is an endemic medicinal tree, the bark of which has been used in Chinese traditional medicine for the treatment of various inflammatory diseases. In our continuing search for bioactive compounds from Korean natural resources, a phytochemical investigation of the bark of B. platyphylla var. japonica led to the isolation of 7-oxo-β-sitosterol (1) and soyacerebroside I (2) from its ethanol extract as main components by liquid chromatography (LC)/mass spectrometry (MS)-based analysis. The structures of isolates were identified by comparison of ¹H and ¹³C nuclear magnetic resonance spectroscopic data and physical data with the previously reported values and LC/MS analyses. To the best of our knowledge, this is the first study to demonstrate that the isolated compounds, 7-oxo-β-sitosterol and soyacerebroside I, were isolated in B. platyphylla var. japonica. We examined the effects of the isolates on the regulation of adipocytes and osteoblast differentiation. These isolates (1 and 2) produced fewer lipid droplets compared to the untreated negative control in Oil Red O staining of the mouse mesenchymal stem cell line without altering the amount of alkaline phosphatase staining. The results demonstrated that both compounds showed marginal inhibitory effects on adipocyte differentiation but did not affect osteoblast differentiation.
Keyword
MeSH Terms
Figure
Reference
-
1. Jiangsu New Medical College. Chinese Material Medica. Shanghai: Shanghai People's Publishing House;1977. p. 1784.2. Kashiwada Y, Sekiya M, Yamazaki K, Ikeshiro Y, Fujioka T, Yamagishi T, Kitagawa S, Takaishi Y. J Nat Prod. 2007; 70:623–627.3. Lee M, Sung SH. . Pharmacogn Mag. 2016; 12:276–281.4. Eom HJ, Kang HR, Choi SU, Kim KH. Chem Biodivers. 2017; 14:e1600400.5. Eom HJ, Kang HR, Kim HK, Jung EB, Park HB, Kang KS, Kim KH. Bioorg Chem. 2016; 66:97–101.6. Kang HR, Yun HS, Lee TK, Lee S, Kim SH, Moon E, Park KM, Kim KH. J Agric Food Chem. 2018; 66:2677–2684.7. Ma XL, Lin WB, Zhang GL. Nat Prod Res Dev. 2009; 21:593–599.8. Tang J, Meng X, Liu H, Zhao J, Zhou L, Qiu M, Zhang X, Yu Z, Yang F. Molecules. 2010; 15:9288–9297.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Clinical Study on the Efficacy of Cosmetics Containing the Root of Ephedra sinica and the Bark of Betula platyphylla var. japonica Related to Skin Furrows
- α-Glucosidase Inhibitory Activity of Phenolic Compounds Isolated from the Stems of Caesalpinia decapetala var. japonica
- The Cytotoxic Constituents of Betula platyphylla and their Effects on Human Lung A549 Cancer Cells
- Transcriptional repression of type I procollagen genes during adipocyte differentiation
- Phytochemical Constituents from the Rhizomes of Osmunda japonica Thunb and Their Anti-oxidant Activity