Nat Prod Sci.
2018 Dec;24(4):219-224. 10.20307/nps.2018.24.4.219.
The Cytotoxic Constituents of Betula platyphylla and their Effects on Human Lung A549 Cancer Cells
- Affiliations
-
- 1Research Institute of Pharmaceutical Sciences, College of Pharmacy, Kyungpook National University, Daegu 41566, Republic of Korea. kssong@knu.ac.kr
- 2Biological and Genetic Resources Assessment Division, National Institute of Biological Resources, Incheon 22689, Republic of Korea.
- KMID: 2432414
- DOI: http://doi.org/10.20307/nps.2018.24.4.219
Abstract
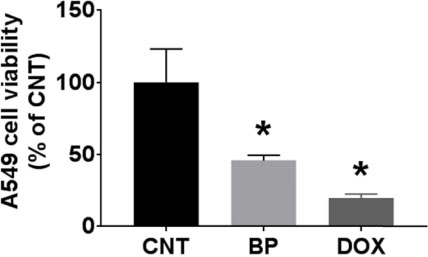
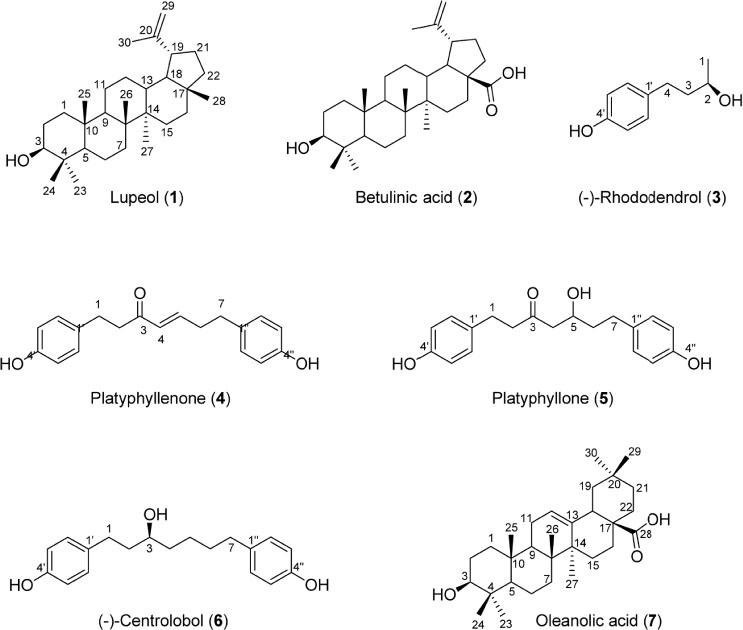
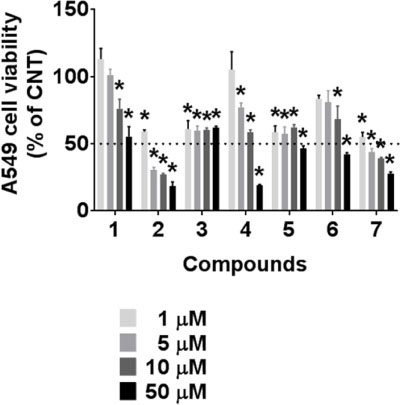
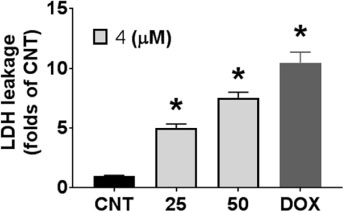
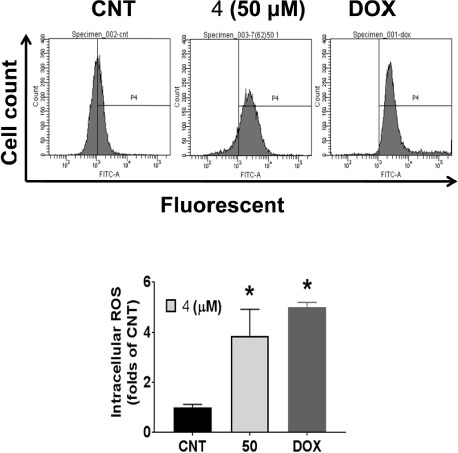
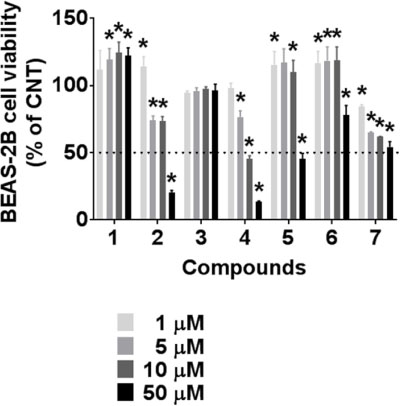
- During the screening for cytotoxic compounds from plants grown in Korea, Betula platyphylla (BP) showed potent activity against the adenocarcinomic human alveolar basal epithelial A549 cell line. To identify the cytotoxic components from BP, the CHâ‚‚Clâ‚‚ fraction with the most significant cytotoxic effect was applied to the column chromatographies. Seven compounds were isolated: lupeol (1), betulinic acid (2), (−)-rhododendrol (3), platyphyllenone (4), platyphyllone (5), (−)-centrolobol (6), and oleanolic acid (7). Among them, three diarylheptanoids (4 - 6) exhibited cytotoxicity toward A549 cells. Especially, 50 µM of 4 reduced A549 cell viability to 18.93 ± 0.82% compared to control (100.00 ± 21.48%). Lactate dehydrogenase (LDH) leakage and intracellular reactive oxygen species (ROS) production were also induced by 50 µM 4. This is the first report on the cytotoxic effect of BP-derived diarylheptanoids 4-6 against A549 cells. The compound 4 may be useful for the development of early hit compounds for non-small cell lung carcinoma, but the consideration about selectivity of 4 is required since 4 also showed the cytotoxicity in the human normal lung epithelial BEAS-2B cell line.
MeSH Terms
Figure
Reference
-
1. Zhu QG, Zhang SM, Ding XX, He B, Zhang HQ. Oncotarget. 2017; 8:57680–57692.2. Dong L, Lei D, Zhang H. Oncotarget. 2017; 8:64600–64606.3. Wang J, Chen J, Guo Y, Wang B, Chu H. Oncotarget. 2017; 8:53854–53872.4. Siegel RL, Miller KD, Jemal A. CA Cancer J Clin. 2016; 66:7–30.5. Zarogoulidis K, Zarogoulidis P, Darwiche K, Boutsikou E, Machairiotis N, Tsakiridis K, Katsikogiannis N, Kougioumtzi I, Karapantzos I, Huang H, Spyratos D. J Thorac Dis. 2013; 5:S4. S389–S396.6. Kroschinsky F, Stölzel F, von Bonin S, Beutel G, Kochanek M, Kiehl M, Schellongowski P. Crit Care. 2017; 21:89.7. Daga A, Ansari A, Patel S, Mirza S, Rawal R, Umrania V. Asian Pac J Cancer Prev. 2015; 16:4147–4156.8. Ancuceanu RV, Istudor V. Altern Med Rev. 2004; 9:402–419.9. Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH, Rollinger JM, Schuster D, Breuss JM, Bochkov V, Mihovilovic MD, Kopp B, Bauer R, Dirsch VM, Stuppner H. Biotechnol Adv. 2015; 33:1582–1614.10. Schelman WR, Mohammed TA, Traynor AM, Kolesar JM, Marnocha RM, Eickhoff J, Keppen M, Alberti DB, Wilding G, Takebe N, Liu G. Invest New Drugs. 2014; 32:295–302.11. Baggstrom MQ, Qi Y, Koczywas M, Argiris A, Johnson EA, Millward MJ, Murphy SC, Erlichman C, Rudin CM, Govindan R. J Thorac Oncol. 2011; 6:1757–1760.12. Ready N, Karaseva NA, Orlov SV, Luft AV, Popovych O, Holmlund JT, Wood BA, Leopold L. J Thorac Oncol. 2011; 6:781–785.13. Eom HJ, Kang HR, Choi SU, Kim KH. Chem Biodivers. 2017; 14.14. Jiang H, Shen Y, Yasuda E, Chiba M, Terazawa M. Eurasian J For Res. 2001; 3:49–54.15. Wang SJ, Pei YH, Hua HM. J Asian Nat Prod Res. 2001; 3:157–160.16. Cho N, Kim HW, Lee HK, Jeon BJ, Sung SH. Biosci Biotechnol Biochem. 2016; 80:166–171.17. Eom HJ, Kang HR, Kim HK, Jung EB, Park HB, Kang KS, Kim KH. Bioorg Chem. 2016; 66:97–101.18. Matsuda H, Ishikado A, Nishida N, Ninomiya K, Fujiwara H, Kobayashi Y, Yoshikawa M. Bioorg Med Chem Lett. 1998; 8:2939–2944.19. Lee M, Park JH, Min DS, Yoo H, Park JH, Kim YC, Sung SH. Biosci Biotechnol Biochem. 2012; 76:1616–1620.20. Lee M, Sung SH. Pharmacogn Mag. 2016; 12:276–281.21. Le CF, Kailaivasan TH, Chow SC, Abdullah Z, Ling SK, Fang CM. Int Immunopharmacol. 2017; 44:203–210.22. Prachayasittikul S, Saraban P, Cherdtrakulkiat R, Ruchirawat S, Prachayasittikul V. EXCLI J. 2010; 9:1–10.23. Kim JH, Byun JC, Bandi AKR, Hyun CG, Lee NH. J Med Plants Res. 2009; 3:914–920.24. Kim MH, Nugroho A, Choi J, Park JH, Park HJ. Arch Pharm Res. 2011; 34:971–978.25. Parmar VS, Vardhan A, Taneja P, Sinha R, Patnaik GK, Tripathi SC, Boll PM, Larsen S. J Chem Soc Perkin Trans 1. 1991; 11:2687–2690.26. Ibrahim SR, Fouad MA, Abdel-Lateff A, Okino T, Mohamed GA. Nat Prod Res. 2014; 28:1765–1771.27. Sunnerheim-Sjöberg K, Knutsson PG. J Chem Ecol. 1995; 21:1339–1348.28. Ohta S, Koyama M, Aoki T, Suga T. Bull Chem Soc Jpn. 1985; 58:2423–2424.29. Onoja E, Ndukwe IG. J Nat Prod Plant Resour. 2013; 3:57–60.30. Awan ZI, Habib-ur-Rehman KAY, Minhas FA. IOSR J Appl Chem. 2013; 5:58–66.31. Fuchino H, Konishi S, Satoh T, Yagi A, Saitsu K, Tatsumi T, Tanaka N. Chem Pharm Bull (Tokyo). 1996; 44:1033–1038.32. Terazawa M, Koga T, Okuyama H, Miyake M. J Jap Wood Res Soc. 1973; 19:47–48.33. Kim SH, Park JH, Kim TB, Lee HH, Lee KY, Kim YC, Sung SH. Bioorg Med Chem Lett. 2010; 20:2824–2827.34. Liao CR, Kuo YH, Ho YL, Wang CY, Yang CS, Lin CW, Chang YS. Molecules. 2014; 19:9515–9534.35. You YJ, Nam NH, Kim Y, Bae KH, Ahn BZ. Phytother Res. 2003; 17:341–344.36. Rao SD, Rao BN, Devi PU, Rao AK. Orient J Chem. 2017; 33:173–180.37. Jang GU, Choi SU, Lee KR. Yakhak Hoeji. 2005; 49:244–248.38. Sidova V, Zoufaly P, Pokorny J, Dzubak P, Hajduch M, Popa I, Urban M. PLoS One. 2017; 12:e0171621.39. Dinić J, Novaković M, Podolski-Renić A, Vajs V, Tešević V, Isaković A, Pešić M. Chem Biol Interact. 2016; 249:36–45.40. Chokchaisiri R, Pimkaew P, Piyachaturawat P, Chalermglin R, Suksamrarn A. Rec Nat Prod. 2014; 8:46–50.41. Tsao SM, Yin MC. J Agric Food Chem. 2015; 63:3196–3204.42. Dong GZ, Jeong JH, Lee YI, Lee SY, Zhao HY, Jeon R, Lee HJ, Ryu JH. Arch Pharm Res. 2017; 40:509–517.43. Tung NH, Kwon HJ, Kim JH, Ra JC, Ding Y, Kim JA, Kim YH. Bioorg Med Chem Lett. 2010; 20:1000–1003.44. Ilic-Tomic T, Sokovic M, Vojnovic S, Ciric A, Veljic M, Nikodinovic-Runic J, Novakovic M. Planta Med. 2017; 83:117–125.45. Park D, Kim HJ, Jung SY, Yook CS, Jin C, Lee YS. Chem Pharm Bull. 2010; 58:238–241.46. Yadav D, Singh SC, Verma RK, Saxena K, Verma R, Murthy PK, Gupta MM. Phytomedicine. 2013; 20:124–132.47. Novaković M, Novaković I, Cvetković M, Sladić D, Tešević V. Braz J Bot. 2015; 38:441–446.48. Novaković M, Pešić M, Trifunović S, Vučković I, Todorović N, Podolski-Renić A, Dinić J, Stojković S, Tešević V, Vajs V, Milosavljević S. Phytochemistr. 2014; 97:46–54.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Mechanism of Interferon-gamma Induced Cytotoxicity on the Lung Cancer Cell Line, A549
- A Clinical Study on the Efficacy of Cosmetics Containing the Root of Ephedra sinica and the Bark of Betula platyphylla var. japonica Related to Skin Furrows
- Salicylate Induced Apoptosis in A549 Cells
- Anti-tumor Activity of Schoenoplectus triqueter Extract by Suppressing the STAT3 Signaling Pathway in A549 Lung Adenocarcinoma Cells
- Antiproliferative effect of Citrus junos extracts on A549 human non-smallcell lung cancer cells