Allergy Asthma Immunol Res.
2019 Mar;11(2):254-266. 10.4168/aair.2019.11.2.254.
A Fusion Protein of Derp2 Allergen and Flagellin Suppresses Experimental Allergic Asthma
- Affiliations
-
- 1Clinical Vaccine R&D Center and Department of Microbiology, Chonnam National University Medical School, Gwangju, Korea. jhrhee@chonnam.ac.kr
- 2Laboratory of In Vivo Molecular imaging, Institute for Molecular Imaging and Theranostics, Chonnam National University Medical School, Gwangju, Korea.
- 3Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea.
- 4Department of Pharmacology and Dental Therapeutics, School of Dentistry, Chonnam National University, Gwangju, Korea. selee@chonnam.ac.kr
- KMID: 2431857
- DOI: http://doi.org/10.4168/aair.2019.11.2.254
Abstract
- PURPOSE
The house dust mite (HDM) is one of the most important sources of indoor allergens and a significant cause of allergic rhinitis and allergic asthma. Our previous studies demonstrated that Vibrio vulnificus flagellin B (FlaB) plus allergen as a co-treatment mixture improved lung function and inhibited eosinophilic airway inflammation through the Toll-like receptor 5 signaling pathway in an ovalbumin (OVA)- or HDM-induced mouse asthma model. In the present study, we fused the major mite allergen Derp2 to FlaB and compared the therapeutic effects of the Derp2-FlaB fusion protein with those of a mixture of Derp2 and FlaB in a Derp2-induced mouse asthma model.
METHODS
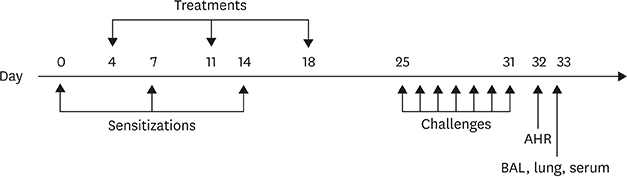
BALB/c mice sensitized with Derp2 + HDM were treated with Derp2, a Derp2 plus FlaB (Derp2 + FlaB) mixture, or the Derp2-FlaB fusion protein 3 times at 1-week intervals. Seven days after the final treatment, the mice were challenged intranasally with Derp2, and airway responses and Derp2-specific immune responses were evaluated.
RESULTS
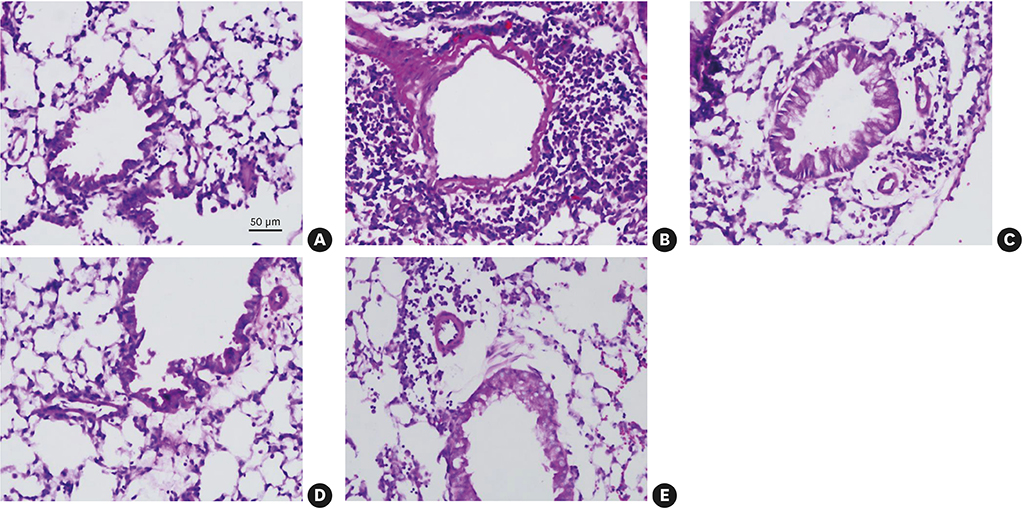
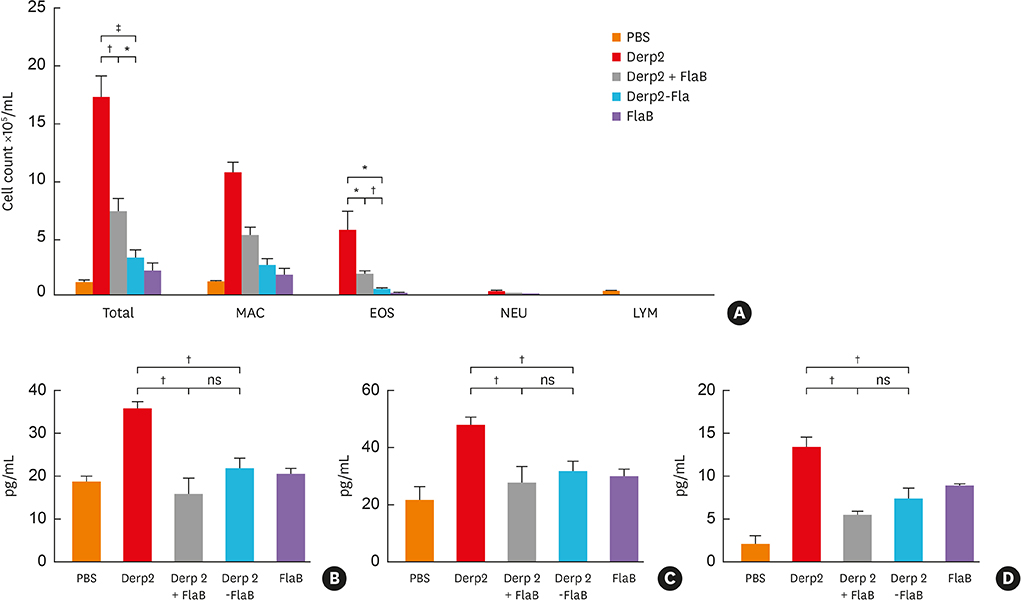
The Derp2-FlaB fusion protein was significantly more efficacious in reducing airway hyperresponsiveness, lung eosinophil infiltration, and Derp2-specific IgE than the Derp2 + FlaB mixture.
CONCLUSIONS
The Derp2-FlaB fusion protein showed a strong anti-asthma immunomodulatory capacity, leading to the prevention of airway inflammatory responses in a murine disease model through the inhibition of Th2 responses. These findings suggest that the Derp2-FlaB fusion protein would be a promising vaccine candidate for HDM-mediated allergic asthma therapy.
Keyword
MeSH Terms
Figure
Reference
-
1. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016; 388:1459–1544.2. Romagnani S. Immunologic influences on allergy and the TH1/TH2 balance. J Allergy Clin Immunol. 2004; 113:395–400.
Article3. Akbari O, Umetsu DT. Role of regulatory dendritic cells in allergy and asthma. Curr Allergy Asthma Rep. 2005; 5:56–61.
Article4. Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. J Allergy Clin Immunol. 2005; 116:961–968.
Article5. Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ J. 2015; 8:17.
Article6. von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010; 10:861–868.
Article7. van Tilburg Bernardes E, Arrieta MC. Hygiene hypothesis in asthma development: is hygiene to blame? Arch Med Res. 2017; 48:717–726.8. Lee SE, Kim SY, Jeong BC, Kim YR, Bae SJ, Ahn OS, et al. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect Immun. 2006; 74:694–702.9. Shim JU, Lee SE, Hwang W, Lee C, Park JW, Sohn JH, et al. Flagellin suppresses experimental asthma by generating regulatory dendritic cells and T cells. J Allergy Clin Immunol. 2016; 137:426–435.
Article10. Shim JU, Rhee JH, Jeong JU, Koh YI. Flagellin modulates the function of invariant NKT cells from patients with asthma via dendritic cells. Allergy Asthma Immunol Res. 2016; 8:206–215.
Article11. Lee SE, Koh YI, Kim MK, Kim YR, Kim SY, Nam JH, et al. Inhibition of airway allergic disease by co-administration of flagellin with allergen. J Clin Immunol. 2008; 28:157–165.
Article12. Thomas WR, Hales BJ, Smith WA. House dust mite allergens in asthma and allergy. Trends Mol Med. 2010; 16:321–328.
Article13. Richardson G, Eick S, Jones R. How is the indoor environment related to asthma?: literature review. J Adv Nurs. 2005; 52:328–339.
Article14. Calderón MA, Linneberg A, Kleine-Tebbe J, De Blay F, Hernandez Fernandez de Rojas D, Virchow JC, et al. Respiratory allergy caused by house dust mites: what do we really know? J Allergy Clin Immunol. 2015; 136:38–48.
Article15. Nguyen CT, Kim SY, Kim MS, Lee SE, Rhee JH. Intranasal immunization with recombinant PspA fused with a flagellin enhances cross-protective immunity against Streptococcus pneumoniae infection in mice. Vaccine. 2011; 29:5731–5739.
Article16. Lee SE, Nguyen CT, Kim SY, Thi TN, Rhee JH. Tetanus toxin fragment C fused to flagellin makes a potent mucosal vaccine. Clin Exp Vaccine Res. 2015; 4:59–67.
Article17. Bordas-Le Floch V, Bussières L, Airouche S, Lautrette A, Bouley J, Berjont N, et al. Expression and characterization of natural-like recombinant Der p 2 for sublingual immunotherapy. Int Arch Allergy Immunol. 2012; 158:157–167.
Article18. Jin HS, Yong TS, Park JW, Hong CS, Oh SH. Immune reactivity of recombinant group 2 allergens of house dust mite, Dermatophagoides pteronyssinus, and Dermatophagoides farinae. J Investig Allergol Clin Immunol. 2003; 13:36–42.19. Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009; 15:410–416.
Article20. Resch Y, Michel S, Kabesch M, Lupinek C, Valenta R, Vrtala S. Different IgE recognition of mite allergen components in asthmatic and nonasthmatic children. J Allergy Clin Immunol. 2015; 136:1083–1091.
Article21. Milián E, Díaz AM. Allergy to house dust mites and asthma. P R Health Sci J. 2004; 23:47–57.22. Cole Johnson C, Ownby DR, Havstad SL, Peterson EL. Family history, dust mite exposure in early childhood, and risk for pediatric atopy and asthma. J Allergy Clin Immunol. 2004; 114:105–110.
Article23. Trombone AP, Tobias KR, Ferriani VP, Schuurman J, Aalberse RC, Smith AM, et al. Use of a chimeric ELISA to investigate immunoglobulin E antibody responses to Der p 1 and Der p 2 in mite-allergic patients with asthma, wheezing and/or rhinitis. Clin Exp Allergy. 2002; 32:1323–1328.24. Li G, Liu Z, Zhong N, Liao B, Xiong Y. Therapeutic effects of DNA vaccine on allergen-induced allergic airway inflammation in mouse model. Cell Mol Immunol. 2006; 3:379–384.25. Li GP, Liu ZG, Qiu J, Ran PX, Zhong NS. DNA vaccine encoding Der p 2 allergen generates immunologic protection in recombinant Der p 2 allergen-induced allergic airway inflammation mice model. Chin Med J (Engl). 2005; 118:534–540.26. Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990; 132:191–195.
Article27. Mizel SB, Honko AN, Moors MA, Smith PS, West AP. Induction of macrophage nitric oxide production by Gram-negative flagellin involves signaling via heteromeric Toll-like receptor 5/Toll-like receptor 4 complexes. J Immunol. 2003; 170:6217–6223.
Article28. Kim JM, Oh YK, Kim YJ, Cho SJ, Ahn MH, Cho YJ. Nuclear factor-kappa B plays a major role in the regulation of chemokine expression of HeLa cells in response to Toxoplasma gondii infection. Parasitol Res. 2001; 87:758–763.
Article29. Koh YI, Shim JU, Lee JH, Chung IJ, Min JJ, Rhee JH, et al. Natural killer T cells are dispensable in the development of allergen-induced airway hyperresponsiveness, inflammation and remodelling in a mouse model of chronic asthma. Clin Exp Immunol. 2010; 161:159–170.
Article30. Schülke S, Burggraf M, Waibler Z, Wangorsch A, Wolfheimer S, Kalinke U, et al. A fusion protein of flagellin and ovalbumin suppresses the TH2 response and prevents murine intestinal allergy. J Allergy Clin Immunol. 2011; 128:1340–1348.e12.
Article31. Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001; 410:1099–1103.
Article32. Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Barrett SL, et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003; 4:1247–1253.
Article33. Repa A, Wild C, Hufnagl K, Winkler B, Bohle B, Pollak A, et al. Influence of the route of sensitization on local and systemic immune responses in a murine model of type I allergy. Clin Exp Immunol. 2004; 137:12–18.
Article34. Gallorini S, Taccone M, Bonci A, Nardelli F, Casini D, Bonificio A, et al. Sublingual immunization with a subunit influenza vaccine elicits comparable systemic immune response as intramuscular immunization, but also induces local IgA and TH17 responses. Vaccine. 2014; 32:2382–2388.
Article35. Hwang HS, Puth S, Tan W, Verma V, Jeong K, Lee SE, et al. More robust gut immune responses induced by combining intranasal and sublingual routes for prime-boost immunization. Hum Vaccin Immunother. 2018; 1–9.
Article36. Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, et al. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003; 171:4984–4989.
Article37. Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, et al. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J Exp Med. 2003; 197:101–109.38. Hajam IA, Dar PA, Shahnawaz I, Jaume JC, Lee JH. Bacterial flagellin-a potent immunomodulatory agent. Exp Mol Med. 2017; 49:e373.
Article39. Bates JT, Uematsu S, Akira S, Mizel SB. Direct stimulation of tlr5+/+ CD11c+ cells is necessary for the adjuvant activity of flagellin. J Immunol. 2009; 182:7539–7547.40. Atif SM, Lee SJ, Li LX, Uematsu S, Akira S, Gorjestani S, et al. Rapid CD4+ T-cell responses to bacterial flagellin require dendritic cell expression of Syk and CARD9. Eur J Immunol. 2015; 45:513–524.41. Tsujimoto H, Uchida T, Efron PA, Scumpia PO, Verma A, Matsumoto T, et al. Flagellin enhances NK cell proliferation and activation directly and through dendritic cell-NK cell interactions. J Leukoc Biol. 2005; 78:888–897.
Article