Yonsei Med J.
2019 Feb;60(2):182-190. 10.3349/ymj.2019.60.2.182.
Silencing the PIK3CA Gene Enhances the Sensitivity of Childhood Leukemia Cells to Chemotherapy Drugs by Suppressing the Phosphorylation of Akt
- Affiliations
-
- 1Department of Pediatric Internal Medicine, Affiliated Hospital of Taishan Medical University, Tai'an, China.
- 2Department of Gynecology, Affiliated Hospital of Taishan Medical University, Tai'an, China. dingmingde24@163.com
- KMID: 2431635
- DOI: http://doi.org/10.3349/ymj.2019.60.2.182
Abstract
- PURPOSE
This study aimed to investigate the effects of PIK3CA on the sensitivity of acute B lymphocytic leukemia cells (Nalm-6 cells) to chemotherapy drugs.
MATERIALS AND METHODS
Children's normal B lymphocytes and Nalm-6 cells were cultured. Nalm-6 cells were transfected with PIK3CA siRNA (siPIK3CA group) or its negative control (PIK3CA-Control group). Normal Nalm-6 cells were named Mock group. Nalm-6 cells transfected by PIK3CA siRNA were treated with Akt inhibitor (siPIK3CA+Akti-1/2 group). mRNA and protein expression was detected by qRT-PCR and Western blot. Proliferation and sensitivity to chemotherapeutic drugs was detected by MTT assay. Cell cycle and apoptosis was explored by low cytometry. Transwell assay was performed to test invasion.
RESULTS
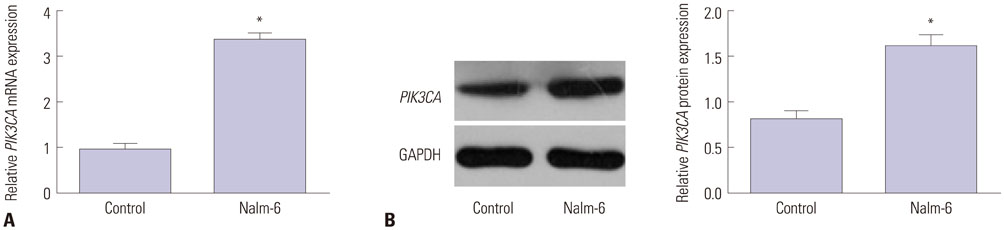
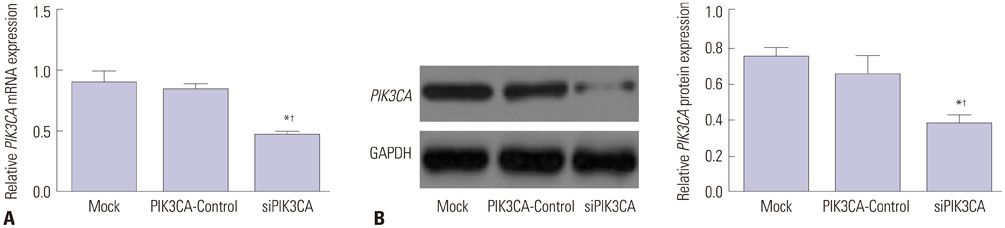
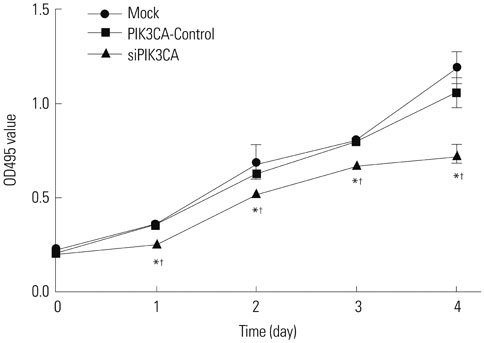
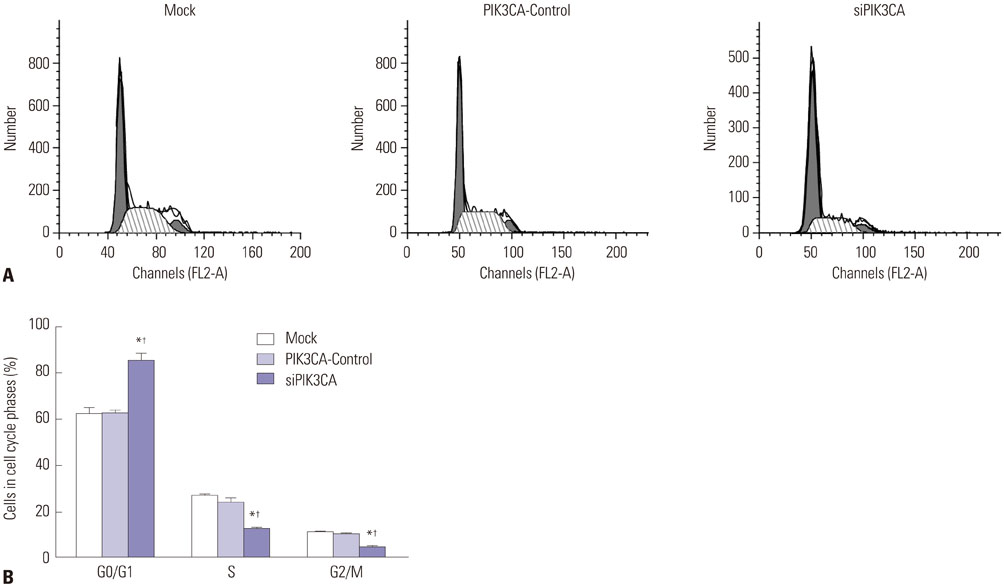
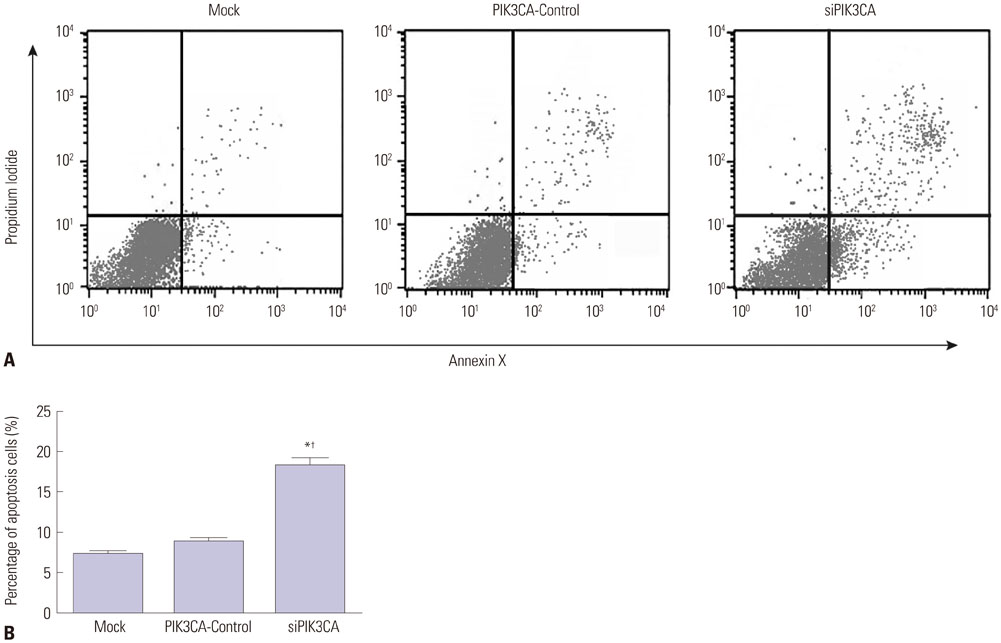
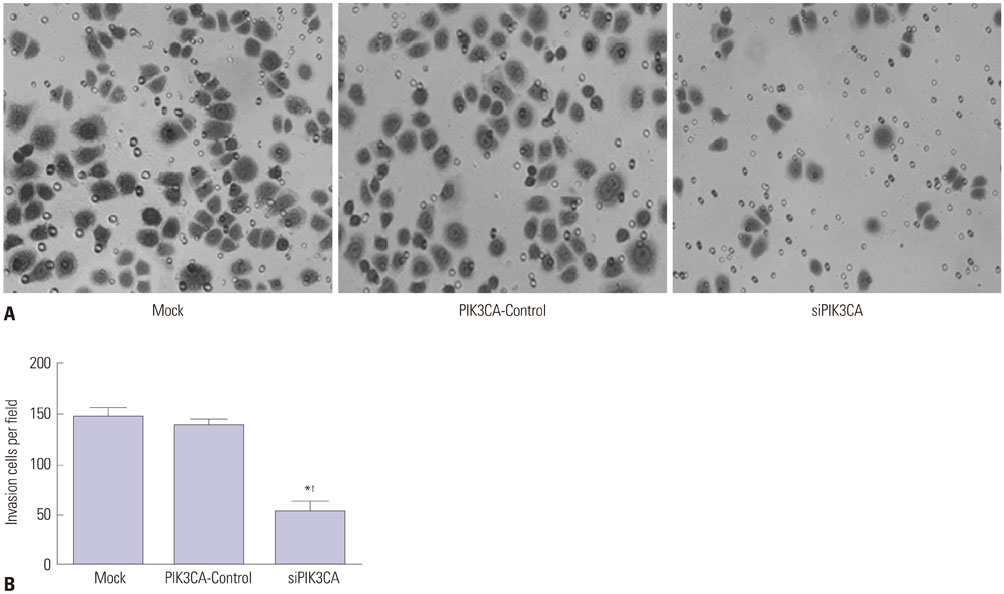
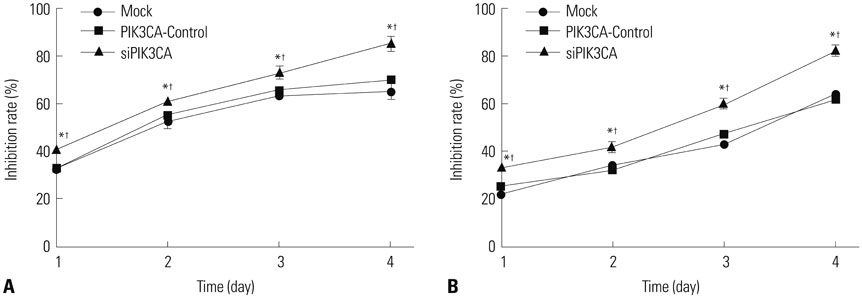
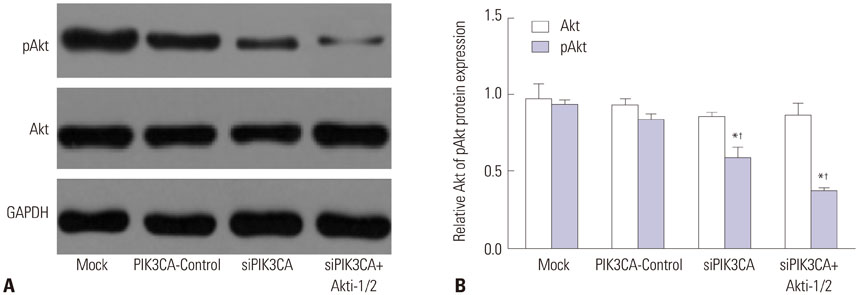
PIK3CA mRNA (p=0.008) and protein (p=0.006) expression was higher in Nalm-6 cells than that in normal B lymphocytes. Compared with the Mock group and PIK3CA-Control group, Nalm-6 cells of the siPIK3CA group had lower OD495 values (all p < 0.05) and invasion cell numbers (p=0.03 and p=0.025), as well as a higher proportion of G0/G1 phase cells (p=0.020 and p=0.022), percentage of apoptosis (p=0.016 and p=0.022), and inhibition rate (all p < 0.05). pAkt expression in the siPIK3CA group (p=0.026 and p=0.031) and siPIK3CA+Akti-1/2 group (p=0.019 and p=0.023) was lower than that in the Mock group.
CONCLUSION
PIK3CA silencing inhibited Nalm-6 cell proliferation and invasion, and promoted their apoptosis and sensitivity to chemotherapeutic drugs, potentially through regulation of the PI3K/AKT signaling pathway.
Keyword
MeSH Terms
Figure
Reference
-
1. Farah RA, Horkos JG, Bustros YD, Farhat HZ, Abla O. A multicenter experience from Lebanon in childhood and adolescent acute myeloid leukemia: high rate of early death in childhood acute promyelocytic leukemia. Mediterr J Hematol Infect Dis. 2015; 7:e2015012.2. Yang R, Zhong L, Yang XQ, Jiang KL, Li L, Song H, et al. Neutrophil elastase enhances the proliferation and decreases apoptosis of leukemia cells via activation of PI3K/Akt signaling. Mol Med Rep. 2016; 13:4175–4182.
Article3. Singh G, Parmar P, Kataria S, Singh S, Sen R. Spectrum of acute and chronic leukemia at a tertiary care hospital, Haryana, India. Int J Res Med Sci. 2016; 4:1115–1118.
Article4. Barrington-Trimis JL, Cockburn M, Metayer C, Gauderman WJ, Wiemels J, McKean-Cowdin R. Trends in childhood leukemia incidence over two decades from 1992 to 2013. Int J Cancer. 2017; 140:1000–1008.
Article5. Janitz AE, Campbell JE, Magzamen S, Pate A, Stoner JA, Peck JD. Traffic-related air pollution and childhood acute leukemia in Oklahoma. Environ Res. 2016; 148:102–111.
Article6. Carew JS, Zhou Y, Albitar M, Carew JD, Keating MJ, Huang P. Mitochondrial DNA mutations in primary leukemia cells after chemotherapy: clinical significance and therapeutic implications. Leukemia. 2003; 17:1437–1447.
Article7. Hsiao YL, Chang PC, Huang HJ, Kuo CC, Chen CY. Treatment of acute lymphoblastic leukemia from traditional chinese medicine. Evid Based Complement Alternat Med. 2014; 2014:601064.
Article8. Rodríguez-Lirio A, Pérez-Yarza G, Fernández-Suárez MR, Alonso-Tejerina E, Boyano MD, Asumendi A. Metformin induces cell cycle arrest and apoptosis in drug-resistant leukemia cells. Leuk Res Treatment. 2015; 2015:516460.
Article9. Macanas-Pirard P, Broekhuizen R, González A, Oyanadel C, Ernst D, García P, et al. Resistance of leukemia cells to cytarabine chemotherapy is mediated by bone marrow stroma, involves cellsurface equilibrative nucleoside transporter-1 removal and correlates with patient outcome. Oncotarget. 2017; 8:23073–23086.
Article10. Wang L, Chen B, Lin M, Cao Y, Chen Y, Chen X, et al. Decreased expression of nucleophosmin/B23 increases drug sensitivity of adriamycin-resistant Molt-4 leukemia cells through mdr-1 regulation and Akt/mTOR signaling. Immunobiology. 2015; 220:331–340.
Article11. Xu H, Li Y, Chen L, Wang C, Wang Q, Zhang H, et al. SIRT2 mediates multidrug resistance in acute myelogenous leukemia cells via ERK1/2 signaling pathway. Int J Oncol. 2016; 48:613–623.
Article12. Jiang Q, Lu X, Huang P, Gao C, Zhao X, Xing T, et al. Expression of miR-652-3p and effect on apoptosis and drug sensitivity in pediatric acute lymphoblastic leukemia. Biomed Res Int. 2018; 2018:5724686.
Article13. Liang H, Zheng QL, Fang P, Zhang J, Zhang T, Liu W, et al. Targeting the PI3K/AKT pathway via GLI1 inhibition enhanced the drug sensitivity of acute myeloid leukemia cells. Sci Rep. 2017; 7:40361.
Article14. Gu M, Nishihara R, Chen Y, Li W, Shi Y, Masugi Y, et al. Aspirin exerts high anti-cancer activity in PIK3CA-mutant colon cancer cells. Oncotarget. 2017; 8:87379–87389.
Article15. Zheng S, Yang C, Lu M, Liu Q, Liu T, Dai F, et al. PIK3CA promotes proliferation and motility but is unassociated with lymph node metastasis or prognosis in esophageal squamous cell carcinoma. Hum Pathol. 2016; 53:121–129.
Article16. Zhang Y, Gao Y, Shi R, Chen D, Wang X, Kamijima M, et al. Household pesticide exposure and the risk of childhood acute leukemia in Shanghai, China. Environ Sci Pollut Res Int. 2015; 22:11755–11763.
Article17. Boles JM, Dutel JL, Briere J, Mialon P, Robasckiewicz M, Garre M, et al. Acute renal failure caused by extreme hyperphosphatemia after chemotherapy of an acute lymphoblastic leukemia. Cancer. 1984; 53:2425–2429.
Article18. Appelbaum FR, Kopecky KJ. Long-term survival after chemotherapy for acute myeloid leukemia: the experience of the Southwest Oncology Group. Cancer. 1997; 80:11 Suppl. 2199–2204.
Article19. Xu P, Wang M, Jiang Y, Ouyang J, Chen B. The association between expression of hypoxia inducible factor-1α and multi-drug resistance of acute myeloid leukemia. Transl Cancer Res. 2017; 6:198–205.
Article20. Gupta R, Chandgothia M, Dahiya M, Bakhshi S, Sharma A, Kumar L. Multi-drug resistance protein 1 as prognostic biomarker in clinical practice for acute myeloid leukemia. Int J Lab Hematol. 2016; 38:e93–e97.
Article21. Rubio MF, Lira MC, Rosa FD, Sambresqui AD, Salazar Güemes MC, Costas MA. RAC3 influences the chemoresistance of colon cancer cells through autophagy and apoptosis inhibition. Cancer Cell Int. 2017; 17:111.
Article22. Bhola PD, Mar BG, Lindsley RC, Ryan JA, Hogdal LJ, Vo TT, et al. Functionally identifiable apoptosis-insensitive subpopulations determine chemoresistance in acute myeloid leukemia. J Clin Invest. 2016; 126:3827–3836.
Article23. Peng Y, Zhang X, Ma Q, Yan R, Qin Y, Zhao Y, et al. MiRNA-194 activates the Wnt/β-catenin signaling pathway in gastric cancer by targeting the negative Wnt regulator, SUFU. Cancer Lett. 2017; 385:117–127.
Article24. Henry WS, Laszewski T, Tsang T, Beca F, Beck AH, McAllister SS, et al. Aspirin suppresses growth in PI3K-mutant breast cancer by activating AMPK and inhibiting mTORC1 signaling. Cancer Res. 2017; 77:790–801.
Article25. Zardavas D, Te Marvelde L, Milne RL, Fumagalli D, Fountzilas G, Kotoula V, et al. Tumor PIK3CA genotype and prognosis in early-stage breast cancer: a pooled analysis of individual patient data. J Clin Oncol. 2018; 36:981–990.
Article26. Cui W, Zheng S, Liu Z, Wang W, Cai Y, Bi R, et al. PIK3CA expression in diffuse large B cell lymphoma tissue and the effect of its knockdown in vitro. Onco Targets Ther. 2017; 10:2239–2247.
Article27. Abubaker J, Bavi PP, Al-Harbi S, Siraj AK, Al-Dayel F, Uddin S, et al. PIK3CA mutations are mutually exclusive with PTEN loss in diffuse large B-cell lymphoma. Leukemia. 2007; 21:2368–2370.
Article28. Burger H, Foekens JA, Look MP, Meijer-van Gelder ME, Klijn JG, Wiemer EA, et al. RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer: correlation with chemotherapeutic response. Clin Cancer Res. 2003; 9:827–836.29. Black JD, Lopez S, Cocco E, Bellone S, Altwerger G, Schwab CL, et al. PIK3CA oncogenic mutations represent a major mechanism of resistance to trastuzumab in HER2/neu overexpressing uterine serous carcinomas. Br J Cancer. 2015; 113:1020–1026.
Article30. Patra S, Young V, Llewellyn L, Senapati JN, Mathew J. BRAF, KRAS and PIK3CA mutation and sensitivity to trastuzumab in breast cancer cell line model. Asian Pac J Cancer Prev. 2017; 18:2209–2213.
Article31. Ross RL, McPherson HR, Kettlewell L, Shnyder SD, Hurst CD, Alder O, et al. PIK3CA dependence and sensitivity to therapeutic targeting in urothelial carcinoma. BMC Cancer. 2016; 16:553.
Article32. Broderick DK, Di C, Parrett TJ, Samuels YR, Cummins JM, McLendon RE, et al. Mutations of PIK3CA in anaplastic oligodendrogliomas, high-grade astrocytomas, and medulloblastomas. Cancer Res. 2004; 64:5048–5050.
Article33. Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999; 274:8347–8350.
Article34. Jiang C, Xu R, Li XX, Wang YY, Liang WQ, Zeng JD, et al. p53R2 overexpression in cervical cancer promotes AKT signaling and EMT, and is correlated with tumor progression, metastasis and poor prognosis. Cell Cycle. 2017; 16:1673–1682.
Article35. Tian B, Chen X, Zhang H, Li X, Wang J, Han W, et al. Urokinase plasminogen activator secreted by cancer-associated fibroblasts induces tumor progression via PI3K/AKT and ERK signaling in esophageal squamous cell carcinoma. Oncotarget. 2017; 8:42300–42313.
Article36. Zhou L, Wu Y, Guo Y, Li Y, Li N, Yang Y, et al. Calycosin enhances some chemotherapeutic drugs inhibition of Akt signaling pathway in gastric cells. Cancer Invest. 2017; 35:289–300.
Article37. Fang WL, Huang KH, Lan YT, Lin CH, Chang SC, Chen MH, et al. Mutations in PI3K/AKT pathway genes and amplifications of PIK3CA are associated with patterns of recurrence in gastric cancers. Oncotarget. 2016; 7:6201–6220.
Article38. Ito C, Nishizuka SS, Ishida K, Uesugi N, Sugai T, Tamura G, et al. Analysis of PIK3CA mutations and PI3K pathway proteins in advanced gastric cancer. J Surg Res. 2017; 212:195–204.
Article39. Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004; 304:554.
Article40. Li Y, Li J. Relationship between PI3K/AKT/PTEN pathway and lung cancer. J Qiqihar Med Coll. 2013; 34:2657–2659.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 12-O-Tetradecanoylphorbol-13-Acetate Induces Keratin 8 Phosphorylation and Reorganization via Expression of Transglutaminase-2
- Maspin Suppresses Survival of Lung Cancer Cells through Modulation of Akt Pathway
- The Difference of Akt Phosphorylation with Different Strengths of Hemodynamic Forces on the Endothelial Cell
- Silencing the livin gene enhances the cytotoxic effects of anticancer drugs on colon cancer cells
- Akt Expression in Acute Leukemia Cells and Its Clinical Significance