Anat Cell Biol.
2018 Dec;51(4):284-291. 10.5115/acb.2018.51.4.284.
Histomorphological changes in the common carotid artery of the male rat in induced hypogonadism
- Affiliations
-
- 1Department of Human Anatomy, University of Nairobi, Nairobi, Kenya. isaacbmn@outlook.com
- KMID: 2430196
- DOI: http://doi.org/10.5115/acb.2018.51.4.284
Abstract
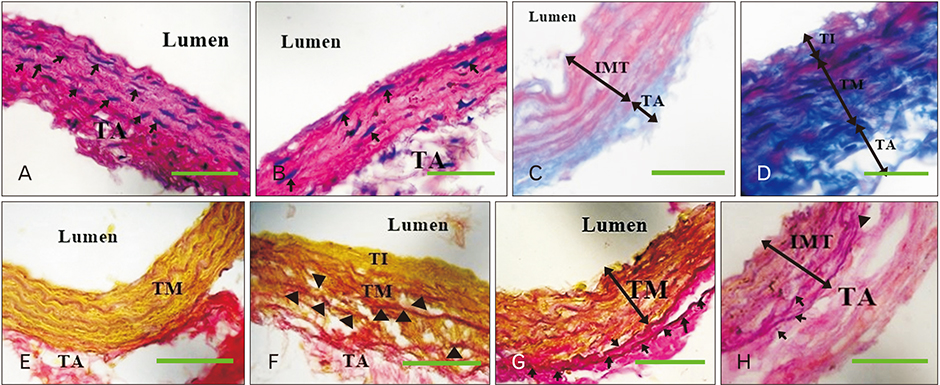
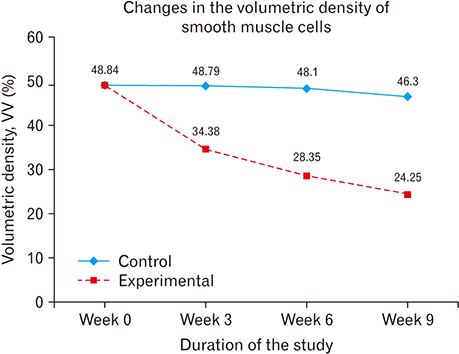
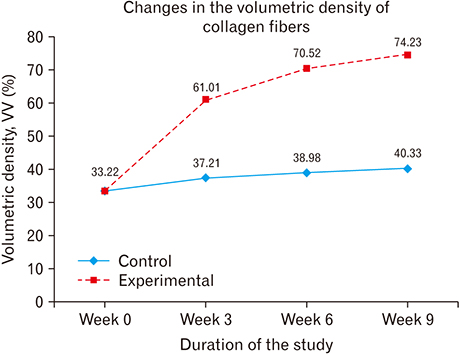
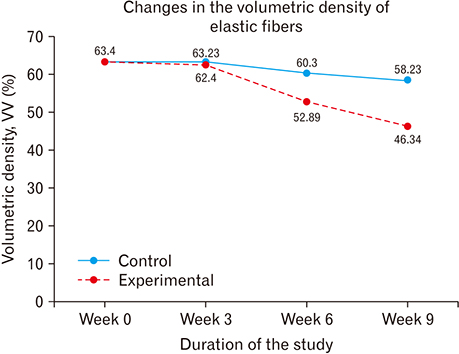
- The role of androgens in the development of cardiovascular diseases remains controversial. The current study therefore sought to determine the changes in the histomorphology of the common carotid artery of the male rat in orchidectomy-induced hypogonadism. Twenty-two Rattus norvegicus male rats aged 2 months were used. The rats were randomly assigned into baseline (n=4), experimental (n=9), and control (n=9) groups. Hypogonadism was surgically induced in the experimental group by bilateral orchiectomy under local anesthesia. At experiment weeks 3, 6, and 9, three rats from each group (experimental and control) were euthanized, their common carotid artery harvested, and routine processing was done for paraffin embedding, sectioning, and staining. The photomicrographs were taken using a digital photomicroscope for morphometric analysis. Orchidectomy resulted in the development of vascular fibrosis, with a significant increase in collagen fiber density and decrease in smooth muscle and elastic fiber density. Moreover, there was development of intimal hyperplasia, with fragmentation of medial elastic lamellae in the common carotid artery of the castrated rats. Orchidectomy induces adverse changes in structure of the common carotid artery of the male rat. These changes may impair vascular function, therefore constituting a possible structural basis for the higher incidences of cardiovascular diseases observed in hypogonadism.
MeSH Terms
Figure
Cited by 1 articles
-
Reversible effect of castration induced hypogonadism on the morphology of the left coronary arteries in adult male rabbits
Duncan Anangwe, Moses Madadi Obimbo, Ibsen Henric Ongidi, Peter Bundi Gichangi
Anat Cell Biol. 2024;57(1):61-69. doi: 10.5115/acb.23.196.
Reference
-
1. McGrath KC, McRobb LS, Heather AK. Androgen therapy and atherosclerotic cardiovascular disease. Vasc Health Risk Manag. 2008; 4:11–21.
Article2. Maravelias C, Dona A, Stefanidou M, Spiliopoulou C. Adverse effects of anabolic steroids in athletes: a constant threat. Toxicol Lett. 2005; 158:167–175.3. Haring R, Baumeister SE, Völzke H, Dörr M, Felix SB, Kroemer HK, Nauck M, Wallaschofski H. Prospective association of low total testosterone concentrations with an adverse lipid profile and increased incident dyslipidemia. Eur J Cardiovasc Prev Rehabil. 2011; 18:86–96.
Article4. Fahed AC, Gholmieh JM, Azar ST. Connecting the lines between hypogonadism and atherosclerosis. Int J Endocrinol. 2012; 2012:793953.
Article5. Yeap BB, Hyde Z, Almeida OP, Norman PE, Chubb SA, Jamrozik K, Flicker L, Hankey GJ. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J Clin Endocrinol Metab. 2009; 94:2353–2359.
Article6. Martín-Merino E, Johansson S, Morris T, García Rodríguez LA. Androgen deprivation therapy and the risk of coronary heart disease and heart failure in patients with prostate cancer: a nested case-control study in UK primary care. Drug Saf. 2011; 34:1061–1077.7. Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A, Day N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007; 116:2694–2701.8. Menke A, Guallar E, Rohrmann S, Nelson WG, Rifai N, Kanarek N, Feinleib M, Michos ED, Dobs A, Platz EA. Sex steroid hormone concentrations and risk of death in US men. Am J Epidemiol. 2010; 171:583–592.9. Karakitsos D, Patrianakos AP, De Groot E, Boletis J, Karabinis A, Kyriazis J, Samonis G, Parthenakis FI, Vardas PE, Daphnis E. Androgen deficiency and endothelial dysfunction in men with end-stage kidney disease receiving maintenance hemodialysis. Am J Nephrol. 2006; 26:536–543.
Article10. Kumar N, Swamy R, Patil J, Guru A, Aithal A, Shetty P. Presence of arteriovenous communication between left testicular vessels and its clinical significance. Case Rep Vasc Med. 2014; 2014:160824.
Article11. Christe N, Meier CA. Hypotestosteronaemia in the aging male: should we treat it? Swiss Med Wkly. 2015; 145:w14216.
Article12. Alexandersen P, Haarbo J, Byrjalsen I, Lawaetz H, Christiansen C. Natural androgens inhibit male atherosclerosis: a study in castrated, cholesterol-fed rabbits. Circ Res. 1999; 84:813–819.13. Muraleedharan V, Jones TH. Testosterone and the metabolic syndrome. Ther Adv Endocrinol Metab. 2010; 1:207–223.14. Bobjer J, Naumovska M, Giwercman YL, Giwercman A. High prevalence of androgen deficiency and abnormal lipid profile in infertile men with non-obstructive azoospermia. Int J Androl. 2012; 35:688–694.
Article15. Castela A, Vendeira P, Costa C. Testosterone, endothelial health, and erectile function. ISRN Endocrinol. 2011; 2011:839149.
Article16. Hanke H, Lenz C, Hess B, Spindler KD, Weidemann W. Effect of testosterone on plaque development and androgen receptor expression in the arterial vessel wall. Circulation. 2001; 103:1382–1385.
Article17. Chan YX, Knuiman MW, Hung J, Divitini ML, Handelsman DJ, Beilby JP, McQuillan B, Yeap BB. Testosterone, dihydrotestosterone and estradiol are differentially associated with carotid intima-media thickness and the presence of carotid plaque in men with and without coronary artery disease. Endocr J. 2015; 62:777–786.
Article18. Farias JM, Tinetti M, Khoury M, Umpierrez GE. Low testosterone concentration and atherosclerotic disease markers in male patients with type 2 diabetes. J Clin Endocrinol Metab. 2014; 99:4698–4703.
Article19. Gilliver SC, Ashworth JJ, Mills SJ, Hardman MJ, Ashcroft GS. Androgens modulate the inflammatory response during acute wound healing. J Cell Sci. 2006; 119(Pt 4):722–732.
Article20. Pelletier G, Lihrmann I, Dubessy C, Luu-The V, Vaudry H, Labrie F. Androgenic down-regulation of urotensin II precursor, urotensin II-related peptide precursor and androgen receptor mRNA in the mouse spinal cord. Neuroscience. 2005; 132:689–696.
Article21. Rodríguez-Moyano M, Díaz I, Dionisio N, Zhang X, Avila-Medina J, Calderón-Sánchez E, Trebak M, Rosado JA, Ordóñez A, Smani T. Urotensin-II promotes vascular smooth muscle cell proliferation through store-operated calcium entry and EGFR transactivation. Cardiovasc Res. 2013; 100:297–306.
Article22. Zhao J, Zhang SF, Shi Y, Ren LQ. Effects of urotensin II and its specific receptor antagonist urantide on rat vascular smooth muscle cells. Bosn J Basic Med Sci. 2013; 13:78–83.
Article23. Morgentaler A. Testosterone deficiency and cardiovascular mortality. Asian J Androl. 2015; 17:26–31.
Article24. Traish A, Kim N. The physiological role of androgens in penile erection: regulation of corpus cavernosum structure and function. J Sex Med. 2005; 2:759–770.25. Olabu BO. Structural changes in the rabbit penile architecture in induced hypogonadism [thesis]. Nairobi: University of Nairobi;2014.26. Sánchez-Más J, Turpín MC, Lax A, Ruipérez JA, Valdés Chávarri M, Pascual-Figal DA. Differential actions of eplerenone and spironolactone on the protective effect of testosterone against cardiomyocyte apoptosis in vitro. Rev Esp Cardiol. 2010; 63:779–787.27. Kang NN, Fu L, Xu J, Han Y, Cao JX, Sun JF, Zheng M. Testosterone improves cardiac function and alters angiotensin II receptors in isoproterenol-induced heart failure. Arch Cardiovasc Dis. 2012; 105:68–76.
Article28. Traish AM, Goldstein I, Kim NN. Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction. Eur Urol. 2007; 52:54–70.
Article29. Ikeda Y, Aihara K, Yoshida S, Sato T, Yagi S, Iwase T, Sumitomo Y, Ise T, Ishikawa K, Azuma H, Akaike M, Kato S, Matsumoto T. Androgen-androgen receptor system protects against angiotensin II-induced vascular remodeling. Endocrinology. 2009; 150:2857–2864.
Article30. Wespes E. Smooth muscle pathology and erectile dysfunction. Int J Impot Res. 2002; 14:Suppl 1. S17–S21.
Article31. Traish AM, Kim N. Weapons of penile smooth muscle destruction: androgen deficiency promotes accumulation of adipocytes in the corpus cavernosum. Aging Male. 2005; 8:141–146.
Article32. Dandona P, Rosenberg MT. A practical guide to male hypogonadism in the primary care setting. Int J Clin Pract. 2010; 64:682–696.
Article33. Rzucidlo EM, Martin KA, Powell RJ. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg. 2007; 45:Suppl A. A25–A32.
Article34. Arnold JT, Isaacs JT. Mechanisms involved in the progression of androgen-independent prostate cancers: it is not only the cancer cell's fault. Endocr Relat Cancer. 2002; 9:61–73.
Article35. Corradi LS, Goes RM, Carvalho HF, Taboga SR. Inhibition of 5-alpha-reductase activity induces stromal remodeling and smooth muscle de-differentiation in adult gerbil ventral prostate. Differentiation. 2004; 72:198–208.36. Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res. 2012; 95:194–204.
Article37. Ailawadi G, Eliason JL, Upchurch GR Jr. Current concepts in the pathogenesis of abdominal aortic aneurysm. J Vasc Surg. 2003; 38:584–588.
Article38. Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006; 12:1075–1080.
Article39. El-Sakka AI. Reversion of penile fibrosis: Current information and a new horizon. Arab J Urol. 2011; 9:49–55.
Article40. Chung CC, Kao YH, Chen YJ, Chen YJ. Androgen modulates cardiac fibrosis contributing to gender differences on heart failure. Aging Male. 2013; 16:22–27.
Article41. Čulić V. Androgens in cardiac fibrosis and other cardiovascular mechanisms. Int J Cardiol. 2015; 179:190–192.
Article42. Ikeda Y, Aihara K, Sato T, Akaike M, Yoshizumi M, Suzaki Y, Izawa Y, Fujimura M, Hashizume S, Kato M, Yagi S, Tamaki T, Kawano H, Matsumoto T, Azuma H, Kato S, Matsumoto T. Androgen receptor gene knockout male mice exhibit impaired cardiac growth and exacerbation of angiotensin II-induced cardiac fibrosis. J Biol Chem. 2005; 280:29661–29666.
Article43. Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007; 257:143–179.
Article44. Chipuk JE, Cornelius SC, Pultz NJ, Jorgensen JS, Bonham MJ, Kim SJ, Danielpour D. The androgen receptor represses transforming growth factor-beta signaling through interaction with Smad3. J Biol Chem. 2002; 277:1240–1248.45. Placencio VR, Sharif-Afshar AR, Li X, Huang H, Uwamariya C, Neilson EG, Shen MM, Matusik RJ, Hayward SW, Bhowmick NA. Stromal transforming growth factor-beta signaling mediates prostatic response to androgen ablation by paracrine Wnt activity. Cancer Res. 2008; 68:4709–4718.46. Petrov VV, Fagard RH, Lijnen PJ. Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension. 2002; 39:258–263.47. Pohlers D, Brenmoehl J, Löffler I, Müller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G. TGF-beta and fibrosis in different organs: molecular pathway imprints. Biochim Biophys Acta. 2009; 1792:746–756.48. Sorescu D. Smad3 mediates angiotensin II- and TGF-beta1-induced vascular fibrosis: Smad3 thickens the plot. Circ Res. 2006; 98:988–989.49. Chow MJ, Turcotte R, Lin CP, Zhang Y. Arterial extracellular matrix: a mechanobiological study of the contributions and interactions of elastin and collagen. Biophys J. 2014; 106:2684–2692.
Article50. Shao JS, Sierra OL, Cohen R, Mecham RP, Kovacs A, Wang J, Distelhorst K, Behrmann A, Halstead LR, Towler DA. Vascular calcification and aortic fibrosis: a bifunctional role for osteopontin in diabetic arteriosclerosis. Arterioscler Thromb Vasc Biol. 2011; 31:1821–1833.51. Iannaccone PM, Jacob HJ. Rats! Dis Model Mech. 2009; 2:206–210.
Article52. Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today. 2007; 81:229–240.
Article53. Sherratt MJ. Tissue elasticity and the ageing elastic fibre. Age (Dordr). 2009; 31:305–325.
Article54. Chung AW, Au Yeung K, Sandor GG, Judge DP, Dietz HC, van Breemen C. Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in the thoracic aortic aneurysm in Marfan syndrome. Circ Res. 2007; 101:512–522.
Article55. Lau AC, Rosenberg H, Duong TT, McCrindle BW, Yeung RS. Elastolytic matrix metalloproteinases and coronary outcome in children with Kawasaki disease. Pediatr Res. 2007; 61:710–715.
Article56. Wagenseil JE, Mecham RP. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res. 2012; 5:264–273.
Article57. Han YP, Tuan TL, Wu H, Hughes M, Garner WL. TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa)B mediated induction of MT1-MMP. J Cell Sci. 2001; 114(Pt 1):131–139.
Article58. Gordon GM, Ledee DR, Feuer WJ, Fini ME. Cytokines and signaling pathways regulating matrix metalloproteinase-9 (MMP-9) expression in corneal epithelial cells. J Cell Physiol. 2009; 221:402–411.
Article59. Traish AM, Guay AT. Are androgens critical for penile erections in humans? Examining the clinical and preclinical evidence. J Sex Med. 2006; 3:382–404.60. Cecelja M, Chowienczyk P. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc Dis. 2012; 1:cvd.2012.012016.
Article61. O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007; 50:1–13.62. Fleenor BS. Large elastic artery stiffness with aging: novel translational mechanisms and interventions. Aging Dis. 2013; 4:76–83.63. Jacob T, Hingorani A, Ascher E. Role of apoptosis and proteolysis in the pathogenesis of iliac artery aneurysms. Vascular. 2005; 13:34–42.
Article64. Isenburg JC, Simionescu DT, Starcher BC, Vyavahare NR. Elastin stabilization for treatment of abdominal aortic aneurysms. Circulation. 2007; 115:1729–1737.
Article65. Oka R, Utsumi T, Endo T, Yano M, Kamijima S, Kamiya N, Shirai K, Suzuki H. Effect of androgen deprivation therapy on arterial stiffness and serum lipid profile changes in patients with prostate cancer: a prospective study of initial 6-month follow-up. Int J Clin Oncol. 2016; 21:389–396.
Article66. Dockery F, Bulpitt CJ, Agarwal S, Vernon C, Rajkumar C. Effect of androgen suppression compared with androgen receptor blockade on arterial stiffness in men with prostate cancer. J Androl. 2009; 30:410–415.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of the Carotid Rupture in Procedure of Tracheostomy
- White Matter Damage and Hippocampal Neurodegeneration Induced by Permanent Bilateral Occlusion of Common Carotid Artery in the Rat: Comparison between Wistar and Sprague-Dawley Strain
- An Anatomical Variant : Low-Lying Bifurcation of the Common Carotid Artery, and Its Surgical Implications in Anterior Cervical Discectomy
- Coexistence of the Absence of the Left Common Carotid Artery, a Common Origin of the Left External Carotid Artery and the Right Common Carotid Artery, and an Aberrant Right Subclavian Artery: A Case Report
- Multiple Carotid Artery Occlusive Diseases Treated with Staged Subclavian-carotid Artery bypass and Carotid Endarterectomy: Case Report