Ann Dermatol.
2016 Feb;28(1):45-54. 10.5021/ad.2016.28.1.45.
Interaction of Wnt5a with Notch1 is Critical for the Pathogenesis of Psoriasis
- Affiliations
-
- 1Department of Dermatology, Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. csesnumd@gmail.com
- 2Department of Dermatology, Hanyang University Hospital, Hanyang University College of Medicine, Seoul, Korea.
- 3Department of Dermatology, Chungnam National University College of Medicine, Daejeon, Korea.
- KMID: 2429481
- DOI: http://doi.org/10.5021/ad.2016.28.1.45
Abstract
- BACKGROUND
Psoriasis is characterized by uncontrolled hyperproliferation, aberrant differentiation, and dermal infiltration of immune cells. Recent studies have reported that Wnt5a and Notch1 signaling are altered in psoriatic skin lesions.
OBJECTIVE
We aimed to investigate the interaction of Wnt5a with Notch 1 with respect to inflammation-mediated epidermal hyperproliferation in psoriasis.
METHODS
Expression of Wnt5a and Notch1 signaling-related proteins were examined in psoriatic skin biopsies. Wnt5a was upregulated in human keratinocytes by treating the cells with its recombinant form (rWnt5a).
RESULTS
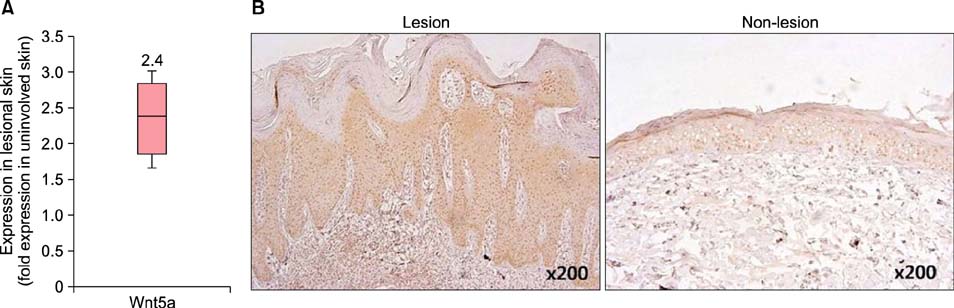
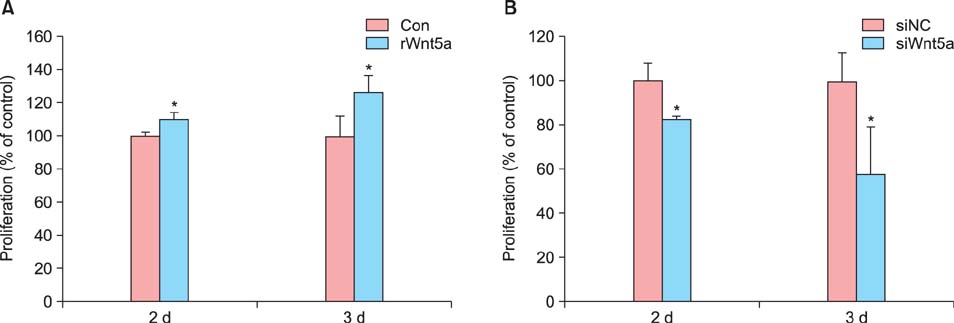
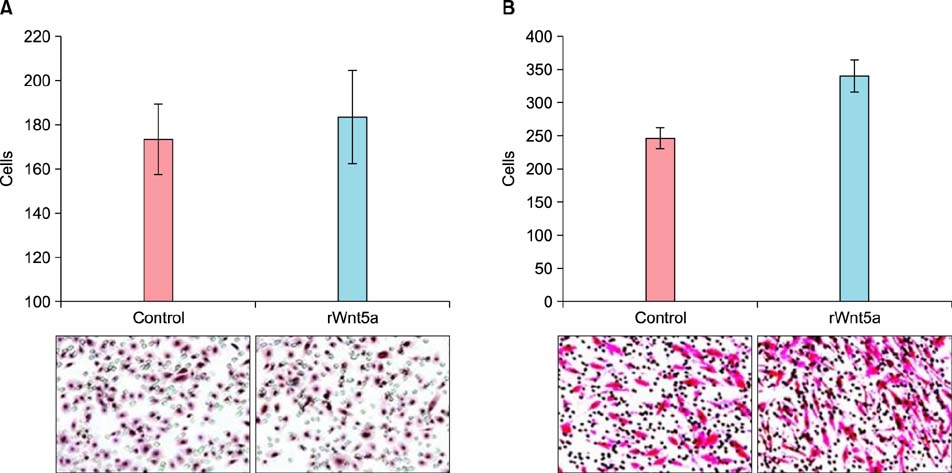
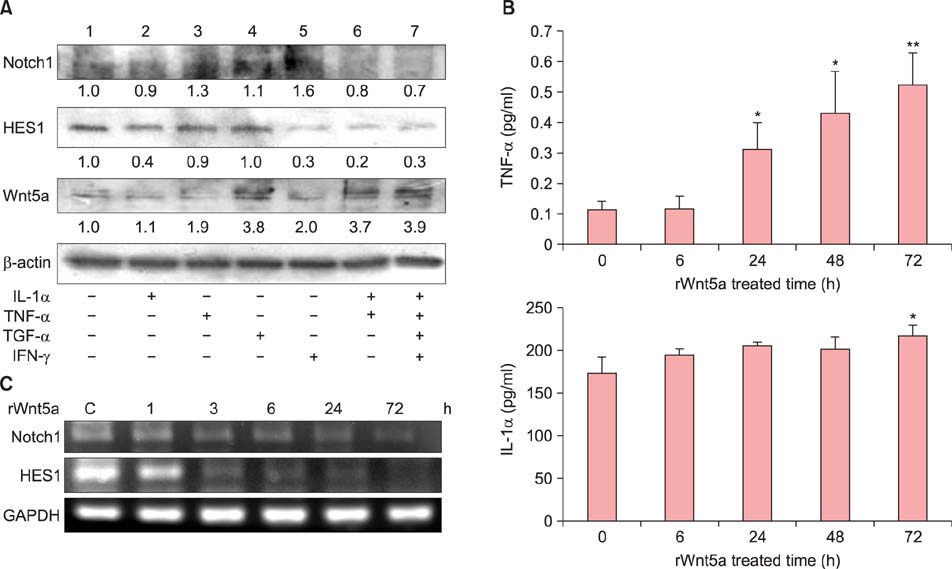
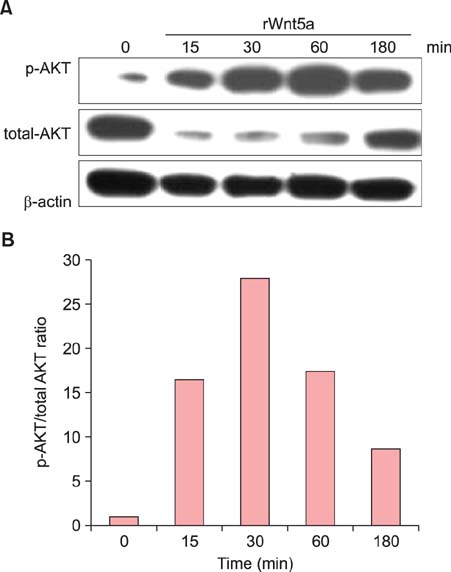
In psoriatic lesions, expression of Wnt5a increased while that of Notch1 decreased when compared to that in non-lesional and normal skin. Treatment with rWnt5a increased the proliferation of keratinocytes and increased their secretion of interleukin (IL)-23, IL-12, and tumor necrosis factor (TNF)-alpha. Further, exposure of keratinocytes to IL-1alpha, TNF-alpha, transforming growth factor-alpha, and interferon-gamma downregulated Notch1 as well as HES 1, which is downstream to Notch1, but increased the Wnt5a levels. The upregulated Wnt5a in keratinocytes downregulated both Notch1 and HES1.
CONCLUSION
Our data suggest that Wnt5a and Notch1 signaling exert counteracting influences on each other and are involved, in part, in the pathomechanism of psoriasis.
MeSH Terms
Figure
Reference
-
1. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009; 361:496–509.
Article2. Reischl J, Schwenke S, Beekman JM, Mrowietz U, Stürzebecher S, Heubach JF. Increased expression of Wnt5a in psoriatic plaques. J Invest Dermatol. 2007; 127:163–169.
Article3. Romanowska M, Evans A, Kellock D, Bray SE, McLean K, Donandt S, et al. Wnt5a exhibits layer-specific expression in adult skin, is upregulated in psoriasis, and synergizes with type 1 interferon. PLoS One. 2009; 4:e5354.
Article4. Gudjonsson JE, Johnston A, Stoll SW, Riblett MB, Xing X, Kochkodan JJ, et al. Evidence for altered Wnt signaling in psoriatic skin. J Invest Dermatol. 2010; 130:1849–1859.
Article5. Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003; 33:416–421.
Article6. Devgan V, Mammucari C, Millar SE, Brisken C, Dotto GP. p21WAF1/Cip1 is a negative transcriptional regulator of Wnt4 expression downstream of Notch1 activation. Genes Dev. 2005; 19:1485–1495.
Article7. Cheng CW, Yeh JC, Fan TP, Smith SK, Charnock-Jones DS. Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem Biophys Res Commun. 2008; 365:285–290.
Article8. Masckauchán TN, Agalliu D, Vorontchikhina M, Ahn A, Parmalee NL, Li CM, et al. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol Biol Cell. 2006; 17:5163–5172.
Article9. Yu JM, Jun ES, Jung JS, Suh SY, Han JY, Kim JY, et al. Role of Wnt5a in the proliferation of human glioblastoma cells. Cancer Lett. 2007; 257:172–181.
Article10. Kawasaki A, Torii K, Yamashita Y, Nishizawa K, Kanekura K, Katada M, et al. Wnt5a promotes adhesion of human dermal fibroblasts by triggering a phosphatidylinositol-3 kinase/Akt signal. Cell Signal. 2007; 19:2498–2506.
Article11. Säfholm A, Leandersson K, Dejmek J, Nielsen CK, Villoutreix BO, Andersson T. A formylated hexapeptide ligand mimics the ability of Wnt-5a to impair migration of human breast epithelial cells. J Biol Chem. 2006; 281:2740–2749.
Article12. Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, et al. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 2006; 108:965–973.
Article13. Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 2008; 28:504–510.
Article14. Mammucari C, Tommasi di Vignano A, Sharov AA, Neilson J, Havrda MC, Roop DR, et al. Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control. Dev Cell. 2005; 8:665–676.
Article15. Nguyen BC, Lefort K, Mandinova A, Antonini D, Devgan V, Della Gatta G, et al. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 2006; 20:1028–1042.
Article16. Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004; 131:965–973.
Article17. Okuyama R, Tagami H, Aiba S. Notch signaling: its role in epidermal homeostasis and in the pathogenesis of skin diseases. J Dermatol Sci. 2008; 49:187–194.
Article18. Thélu J, Rossio P, Favier B. Notch signalling is linked to epidermal cell differentiation level in basal cell carcinoma, psoriasis and wound healing. BMC Dermatol. 2002; 2:7.
Article19. Iizuka H, Takahashi H, Honma M, Ishida-Yamamoto A. Unique keratinization process in psoriasis: late differentiation markers are abolished because of the premature cell death. J Dermatol. 2004; 31:271–276.
Article20. Okuyama R, Ogawa E, Nagoshi H, Yabuki M, Kurihara A, Terui T, et al. p53 homologue, p51/p63, maintains the immaturity of keratinocyte stem cells by inhibiting Notch1 activity. Oncogene. 2007; 26:4478–4488.
Article21. Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001; 20:3427–3436.
Article22. Blaumueller CM, Qi H, Zagouras P, Artavanis-Tsakonas S. Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell. 1997; 90:281–291.
Article23. Kim JE, Lee JH, Jeong KH, Kim GM, Kang H. Notch intracellular domain expression in various skin fibroproliferative diseases. Ann Dermatol. 2014; 26:332–337.
Article24. Collins BJ, Kleeberger W, Ball DW. Notch in lung development and lung cancer. Semin Cancer Biol. 2004; 14:357–364.
Article25. Okuyama R, Nguyen BC, Talora C, Ogawa E, Tommasi di Vignano A, Lioumi M, et al. High commitment of embryonic keratinocytes to terminal differentiation through a Notch1-caspase 3 regulatory mechanism. Dev Cell. 2004; 6:551–562.
Article26. Lefort K, Mandinova A, Ostano P, Kolev V, Calpini V, Kolfschoten I, et al. Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKalpha kinases. Genes Dev. 2007; 21:562–577.
Article27. Nickoloff BJ, Qin JZ, Chaturvedi V, Denning MF, Bonish B, Miele L. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 2002; 9:842–855.
Article28. Ota T, Takekoshi S, Takagi T, Kitatani K, Toriumi K, Kojima T, et al. Notch signaling may be involved in the abnormal differentiation of epidermal keratinocytes in psoriasis. Acta Histochem Cytochem. 2014; 47:175–183.
Article29. Rooney P, Connolly M, Gao W, McCormick J, Biniecka M, Sullivan O, et al. Notch-1 mediates endothelial cell activation and invasion in psoriasis. Exp Dermatol. 2014; 23:113–118.
Article30. Zhou X, Krueger JG, Kao MC, Lee E, Du F, Menter A, et al. Novel mechanisms of T-cell and dendritic cell activation revealed by profiling of psoriasis on the 63,100-element oligonucleotide array. Physiol Genomics. 2003; 13:69–78.
Article31. van Amerongen R, Mikels A, Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci Signal. 2008; 1:re9.
Article32. Couso JP, Martinez Arias A. Notch is required for wingless signaling in the epidermis of Drosophila. Cell. 1994; 79:259–272.
Article33. Ayyanan A, Civenni G, Ciarloni L, Morel C, Mueller N, Lefort K, et al. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci U S A. 2006; 103:3799–3804.
Article34. Ann EJ, Kim HY, Seo MS, Mo JS, Kim MY, Yoon JH, et al. Wnt5a controls Notch1 signaling through CaMKII-mediated degradation of the SMRT corepressor protein. J Biol Chem. 2012; 287:36814–36829.
Article35. Katoh M, Katoh M. Transcriptional mechanisms of WNT5A based on NF-kappaB, Hedgehog, TGFbeta, and Notch signaling cascades. Int J Mol Med. 2009; 23:763–769.36. Koyanagi M, Bushoven P, Iwasaki M, Urbich C, Zeiher AM, Dimmeler S. Notch signaling contributes to the expression of cardiac markers in human circulating progenitor cells. Circ Res. 2007; 101:1139–1145.
Article37. Zhu X, Wu Y, Huang S, Chen Y, Tao Y, Wang Y, et al. Overexpression of Wnt5a in mouse epidermis causes no psoriasis phenotype but an impairment of hair follicle anagen development. Exp Dermatol. 2014; 23:926–928.
Article38. Kim JE, Won CH, Bak H, Kositratna G, Manstein D, Dotto GP, et al. Gene profiling analysis of the early effects of ablative fractional carbon dioxide laser treatment on human skin. Dermatol Surg. 2013; 39:1033–1043.
Article39. Suárez-Fariñas M, Fuentes-Duculan J, Lowes MA, Krueger JG. Resolved psoriasis lesions retain expression of a subset of disease-related genes. J Invest Dermatol. 2011; 131:391–400.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case showing Improved Psoriasis along the Hemiparetic area after Cerebrovascular Accident
- Potential biomarkers and signaling pathways associated with the pathogenesis of primary salivary gland carcinoma: a bioinformatics study
- Coexistence of Bullous Pemphigoid and Psoriasis: A Case Report and Review of the Literature
- Significance of Notch Expression in Acute Myeloid Leukemia
- Immunohistochemical studies of Interleukin-8 and Tumor necrosis factor-alpha on psoriatic lesions