Blood Res.
2018 Dec;53(4):299-306. 10.5045/br.2018.53.4.299.
Neutrophil oxidative burst as a diagnostic indicator of IgG-mediated anaphylaxis
- Affiliations
-
- 1Department of Clinical Pathology, School of Medicine, Kyungpook National University, Daegu, Korea. wondi@knu.ac.kr
- 2Division of Allergy and Infectious Diseases, Department of Internal Medicine, School of Medicine, Kyungpook National University, Daegu, Korea.
- 3Green Cross Reference Laboratory, Yongin, Korea.
- KMID: 2429304
- DOI: http://doi.org/10.5045/br.2018.53.4.299
Abstract
- BACKGROUND
IgG-mediated anaphylaxis occurs after infusion of certain monoclonal antibody-based therapeutics. New in vitro tests are urgently needed to diagnose such reactions. We investigated whether allergens trigger neutrophil oxidative burst (OB) and if neutrophil OB occurs due to allergen-specific IgG (sIgG).
METHODS
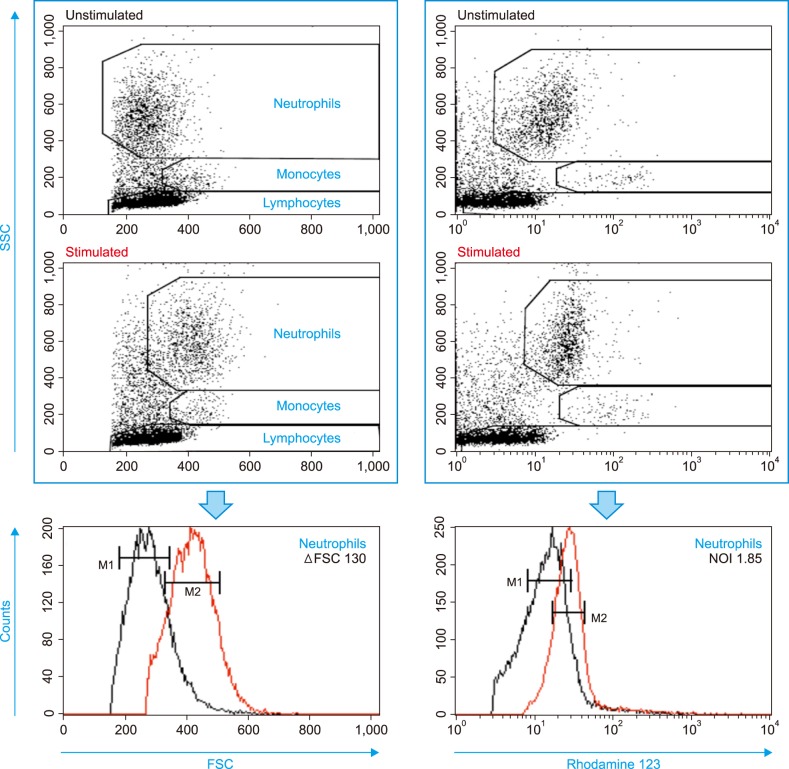
Neutrophil OB was measured by dihydrorhodamine 123 flow cytometry using a leukocyte suspension spiked with a very small patch of the allergen crude extract, Dermatophagoides farinae (Der f). The mean fluorescence intensity ratio of stimulated to unstimulated samples was calculated as the neutrophil oxidative index (NOI).
RESULTS
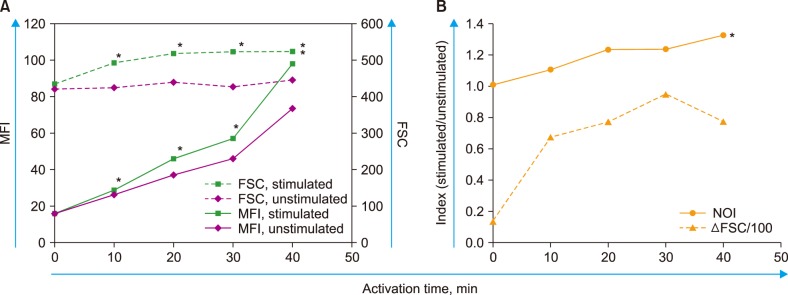
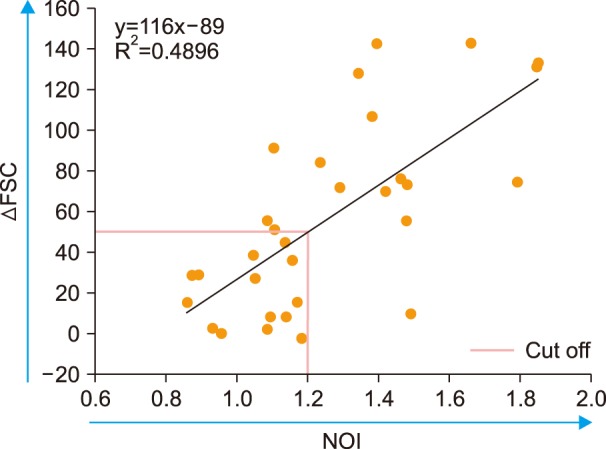
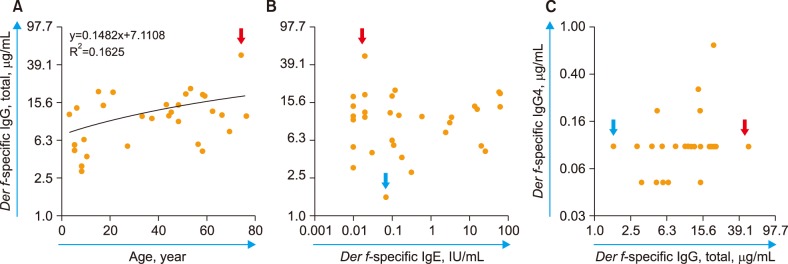
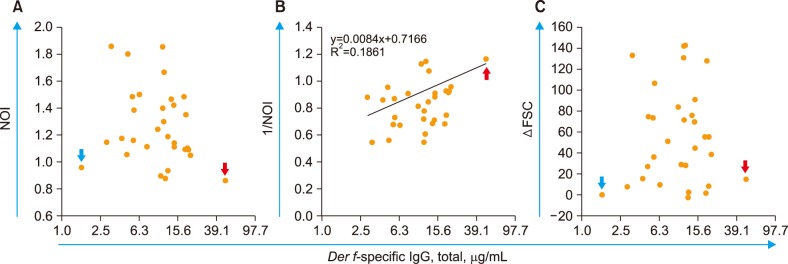
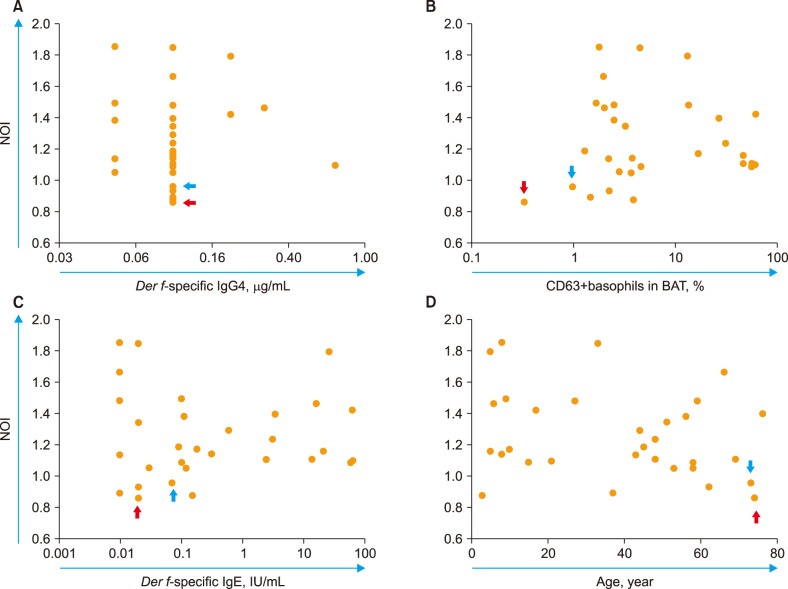
The Der f-specific NOI (Der f-sNOI) showed a time-dependent increase after Der f extract addition. At 15 min activation, higher Der f-sIgG levels were associated with lower Der f-sNOI values in 31 subjects (P < 0.05). This inverse relationship occurs due to the initial blocking effect of free Der f-sIgG. Additionally, neutrophil OB was nearly absent (Der f-sNOI of −1) in two cases: a subject with undetectable Der f-sIgG levels and washed leukocyte suspensions deprived of Der f-sIgG.
CONCLUSION
Allergens can trigger neutrophil OB via preexisting allergen-sIgG. Neutrophil OB can be easily measured in a leukocyte suspension spiked with the allergen. This assay can be used to diagnose IgG-mediated anaphylaxis.
MeSH Terms
Figure
Reference
-
1. Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007; 120:506–515. PMID: 17765751.
Article2. Muñoz-Cano R, Picado C, Valero A, Bartra J. Mechanisms of anaphylaxis beyond IgE. J Investig Allergol Clin Immunol. 2016; 26:73–82.
Article3. Finkelman FD, Khodoun MV, Strait R. Human IgE-independent systemic anaphylaxis. J Allergy Clin Immunol. 2016; 137:1674–1680. PMID: 27130857.
Article4. Escribese MM, Rosace D, Chivato T, Fernández TD, Corbí AL, Barber D. Alternative anaphylactic routes: the potential role of macrophages. Front Immunol. 2017; 8:515. PMID: 28533777.
Article5. Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest. 2006; 116:833–841. PMID: 16498503.6. Jönsson F, Mancardi DA, Kita Y, et al. Mouse and human neutrophils induce anaphylaxis. J Clin Invest. 2011; 121:1484–1496. PMID: 21436586.
Article7. Jönsson F, Mancardi DA, Zhao W, et al. Human FcγRIIA induces anaphylactic and allergic reactions. Blood. 2012; 119:2533–2544. PMID: 22138510.
Article8. Kajiwara N, Sasaki T, Bradding P, et al. Activation of human mast cells through the platelet-activating factor receptor. J Allergy Clin Immunol. 2010; 125:1137–1145. PMID: 20392487.
Article9. Vadas P, Gold M, Perelman B, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008; 358:28–35. PMID: 18172172.
Article10. Montañez MI, Mayorga C, Bogas G, et al. Epidemiology, mechanisms, and diagnosis of drug-induced anaphylaxis. Front Immunol. 2017; 8:614. PMID: 28611774.
Article11. Khodoun MV, Strait R, Armstrong L, Yanase N, Finkelman FD. Identification of markers that distinguish IgE- from IgG-mediated anaphylaxis. Proc Natl Acad Sci U S A. 2011; 108:12413–12418. PMID: 21746933.
Article12. Walrand S, Valeix S, Rodriguez C, Ligot P, Chassagne J, Vasson MP. Flow cytometry study of polymorphonuclear neutrophil oxidative burst: a comparison of three fluorescent probes. Clin Chim Acta. 2003; 331:103–110. PMID: 12691870.
Article13. Robinson JP, Carter WO. Flow cytometric analysis of granulocytes. In : Bauer KD, Duque RE, Shankey TV, editors. Clinical flow cytometry: principles and application. 2nd ed. Baltimore, MD: Williams & Wilkins;1993. p. 405–433.14. Richardson MP, Ayliffe MJ, Helbert M, Davies EG. A simple flow cytometry assay using dihydrorhodamine for the measurement of the neutrophil respiratory burst in whole blood: comparison with the quantitative nitrobluetetrazolium test. J Immunol Methods. 1998; 219:187–193. PMID: 9831400.
Article15. Kim Z, Choi BS, Kim JK, Won DI. Basophil markers for identification and activation in the indirect basophil activation test by flow cytometry for diagnosis of autoimmune urticaria. Ann Lab Med. 2016; 36:28–35. PMID: 26522756.
Article16. Park HJ, Lee JH, Park KH, et al. A nationwide survey of inhalant allergens sensitization and levels of indoor major allergens in Korea. Allergy Asthma Immunol Res. 2014; 6:222–227. PMID: 24843797.
Article17. Hirai T, Yoshioka Y, Takahashi H, et al. High-dose cutaneous exposure to mite allergen induces IgG-mediated protection against anaphylaxis. Clin Exp Allergy. 2016; 46:992–1003. PMID: 26892276.
Article18. Fossati G, Bucknall RC, Edwards SW. Insoluble and soluble immune complexes activate neutrophils by distinct activation mechanisms: changes in functional responses induced by priming with cytokines. Ann Rheum Dis. 2002; 61:13–19. PMID: 11779751.
Article19. Coxon A, Cullere X, Knight S, et al. Fc gamma RIII mediates neutrophil recruitment to immune complexes. a mechanism for neutrophil accumulation in immune-mediated inflammation. Immunity. 2001; 14:693–704. PMID: 11420040.20. Zhang W, Voice J, Lachmann PJ. A systematic study of neutrophil degranulation and respiratory burst in vitro by defined immune complexes. Clin Exp Immunol. 1995; 101:507–514. PMID: 7664498.
Article21. Rojas E, Llinas P, Rodríguez-Romero A, et al. Hevein, an allergenic lectin from rubber latex, activates human neutrophils' oxidative burst. Glycoconj J. 2001; 18:339–345. PMID: 11788802.22. Shouval D, Roggendorf H, Roggendorf M. Enhanced immune response to hepatitis B vaccination through immunization with a Pre-S1/Pre-S2/S vaccine. Med Microbiol Immunol. 2015; 204:57–68. PMID: 25557605.
Article23. Doener F, Michel A, Reuter S, et al. Mast cell-derived mediators promote murine neutrophil effector functions. Int Immunol. 2013; 25:553–561. PMID: 23728776.
Article24. Jönsson F, Mancardi DA, Albanesi M, Bruhns P. Neutrophils in local and systemic antibody-dependent inflammatory and anaphylactic reactions. J Leukoc Biol. 2013; 94:643–656. PMID: 23532517.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Polymorphonuclear leukocyte functions enhanced by chemotaxis
- Age-Related Decrease and a Simple Flow Cytometric Assay of Neutrophil Function
- Human Amniotic Epithelial Cells Affect the Functions of Neutrophils
- Effect of the inhibition of PLA2 on the oxidative stress in the lungs of glutathione depleted rats given endotoxin intratracheally
- Anti-IL-4 antibody inhibits antigen specific IgE response but fails to prevent chicken gamma globulin-induced active systemic anaphylaxis: evidence for the involvement of IgG antibodies