Int J Stem Cells.
2020 Jul;13(2):212-220. 10.15283/ijsc19155.
Human Amniotic Epithelial Cells Affect the Functions of Neutrophils
- Affiliations
-
- 1Department of Immunology, Medical School, Isfahan University of Medical Sciences, Isfahan, Iran
- 2Department of Immunology, Autoimmune Diseases Research Center, Kashan University of Medical Sciences, Kashan, Iran
- 3Department of Immunology, Asadabad School of Medical Science, Asadabad, Iran
- 4Cellular and Molecular Research Center, Department of Immunology, Medical School, Birjand University of Medical Sciences, Birjand, Iran
- KMID: 2504334
- DOI: http://doi.org/10.15283/ijsc19155
Abstract
- Background and Objectives
As a stem cell group, Human amniotic epithelial cells (HAECs) have numerous advantages over their embryonic and adult counterparts for therapeutic utility. They are closer to clinical applications compared to other stem cell types. Additionally, the anti-inflammatory and immunoregulatory properties of HAECs toward several immune cells have been shown previously. Nevertheless, despite the ever-increasing importance of neutrophils in the immune and non-immune processes, a few studies investigated the interaction of neutrophils and HAECs. To increase the current knowledge of HAECs immunology which is necessary for optimizing their future clinical applications, here we explored the effect of HAECs on two chief neutrophil functions; respiratory burst and phagocytosis.
Methods and Results
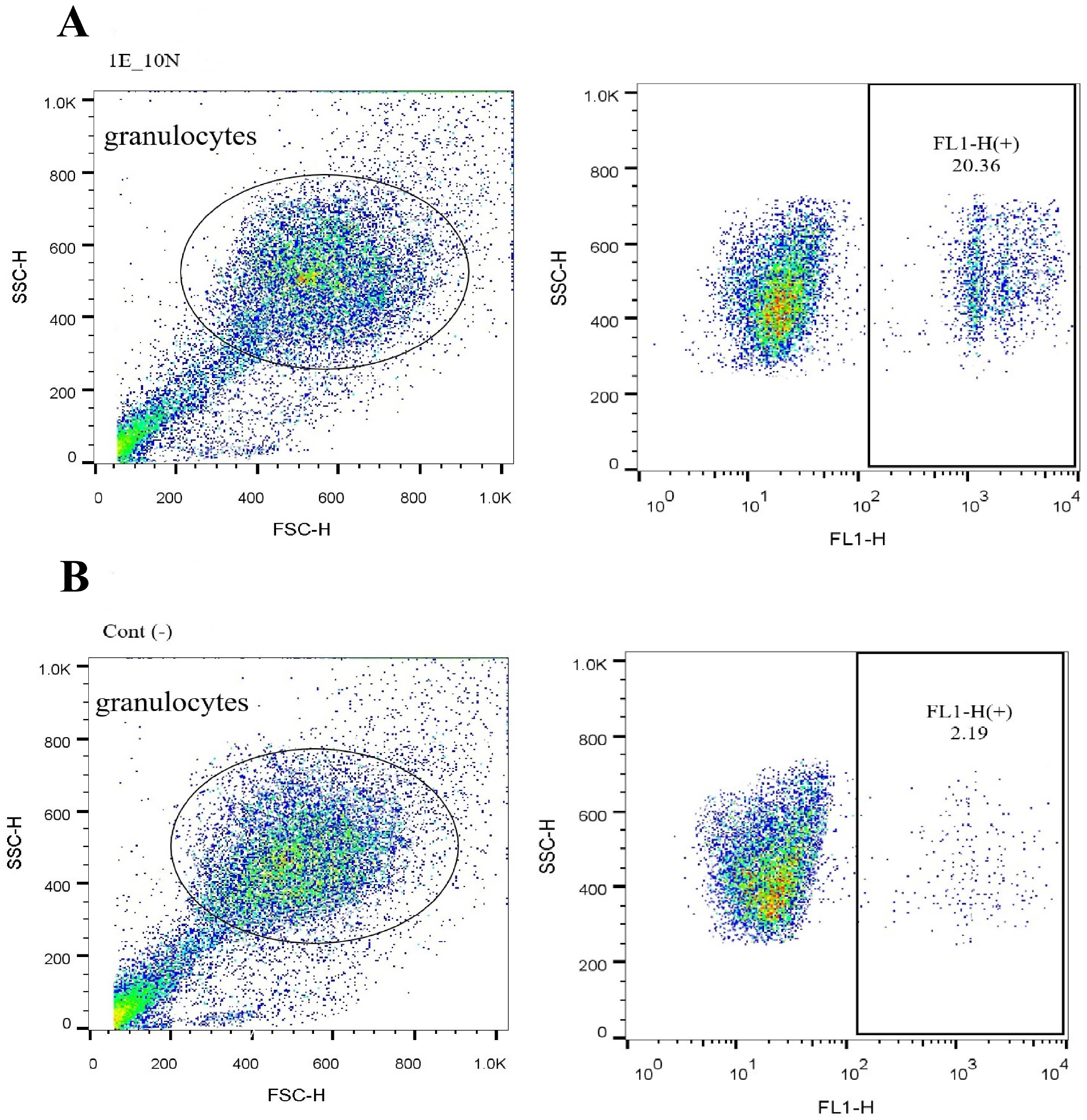
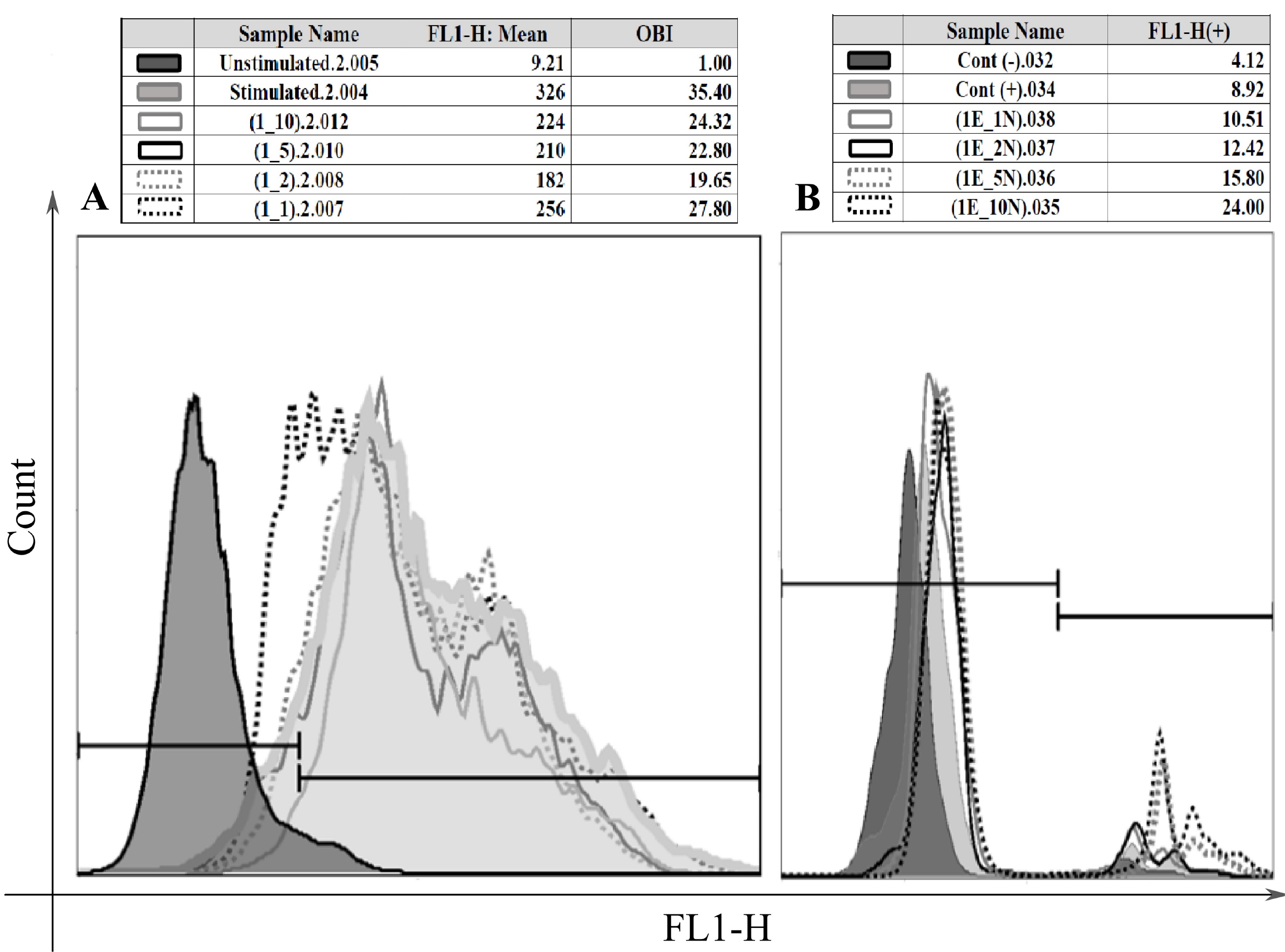
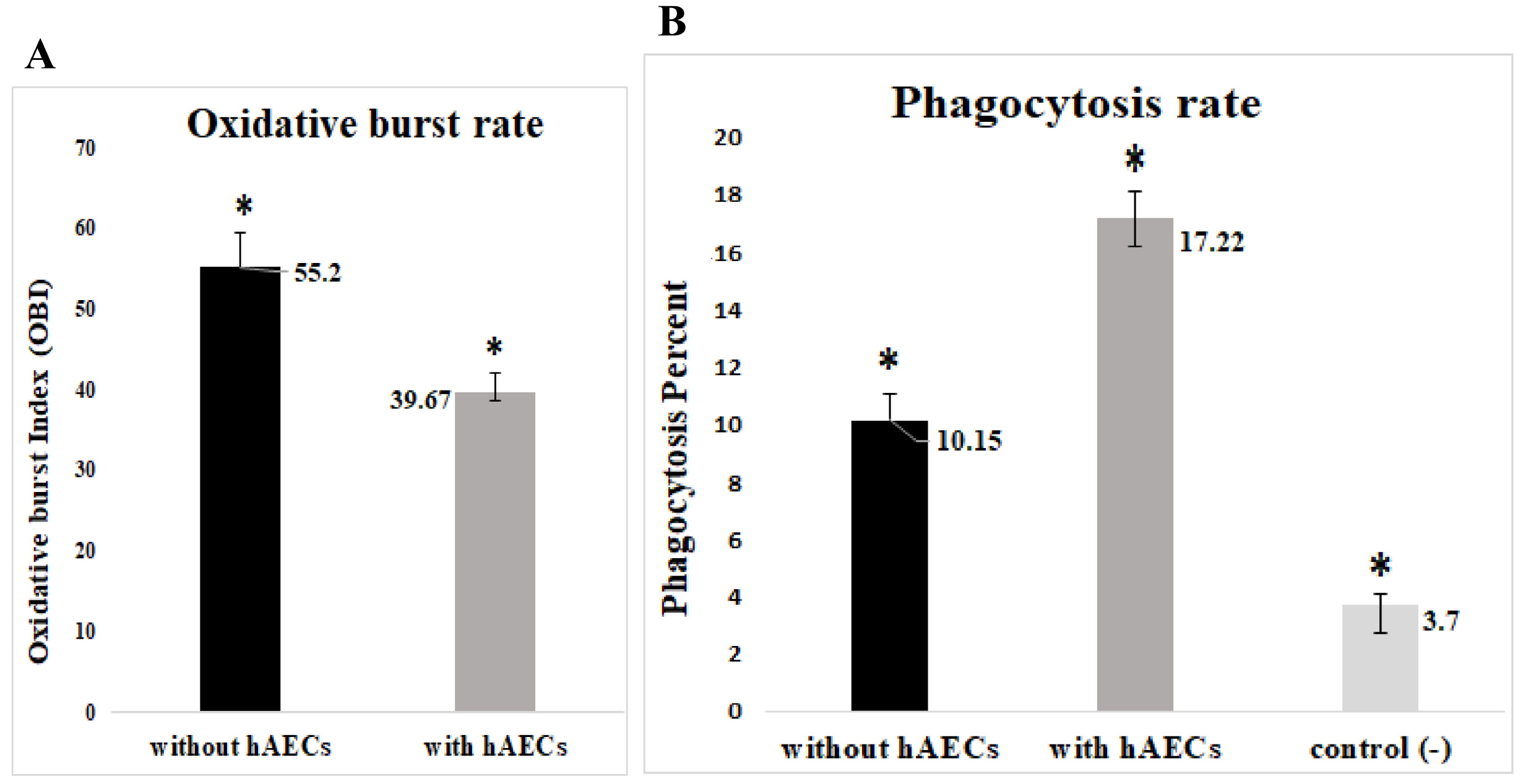
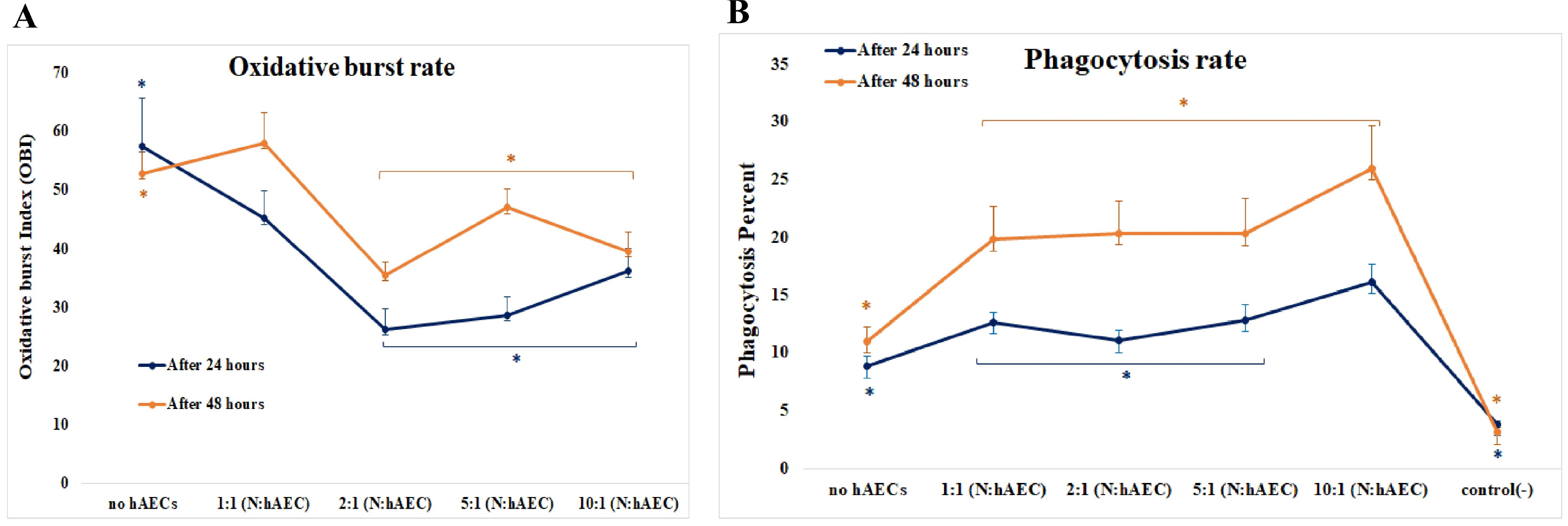
Freshly isolated human blood neutrophils were co-cultured with different number of HAECs for about 24 or 48 hours, then the oxidative burst and phagocytosis of stimulated neutrophils were assessed and compared. The results demonstrated a substantial elevation in the phagocytosis percentage, conversely a significant reduction in the oxidative burst of HAECs-cocultured neutrophils. These effects were dose-dependent, but did not show similar patterns. Likewise, the elongation of coculture period inversely influenced the HAECs-induced effects on the two neutrophil functions.
Conclusions
The present study, for the first time, investigated the HAECs-mediated effects on the two main neutrophil functions. The findings suggest that HAECs by enhancement of phagocytic ability and simultaneously, attenuation of oxidative burst capacity of neutrophils protect the fetus from both microbial treats and oxidative stress and their consequent inflammation; thus corroborate the current anti-inflammatory vision of HAECs.
Figure
Cited by 1 articles
-
Therapeutic Aspects of Mesenchymal Stem Cell-Based Cell Therapy with a Focus on Human Amniotic Epithelial Cells in Multiple Sclerosis: A Mechanistic Review
Reza ArefNezhad, Hossein Motedayyen, Ali Mohammadi
Int J Stem Cells. 2021;14(3):241-251. doi: 10.15283/ijsc21032.
Reference
-
References
1. de la Torre P, Pérez-Lorenzo MJ, Flores AI. Valarmathi MT, editor. 2019. Human placenta-derived mesenchymal stromal cells: a review from basic research to clinical applications. Stromal Cells. IntechOpen;London: p. 230–231. DOI: 10.5772/intechopen.76718.
Article2. Miki T. 2016; A rational strategy for the use of amniotic epithelial stem cell therapy for liver diseases. Stem Cells Transl Med. 5:405–409. DOI: 10.5966/sctm.2015-0304. PMID: 26941361. PMCID: PMC4798744.
Article3. Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. 2007; Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 77:577–588. DOI: 10.1095/biolreprod.106.055244. PMID: 17494917.
Article4. Miki T. 2018; Stem cell characteristics and the therapeutic potential of amniotic epithelial cells. Am J Reprod Immunol. 80:e13003. DOI: 10.1111/aji.13003. PMID: 29956869.
Article5. Motedayyen H, Fathi F, Fasihi-Ramandi M, Ali Taheri R. 2018; The effect of lipopolysaccharide on anti-inflammatory and pro-inflammatory cytokines production of human amniotic epithelial cells. Reprod Biol. 18:404–409. DOI: 10.1016/j.repbio.2018.09.005. PMID: 30220549.
Article6. Magatti M, Cargnoni A, Silini A, Parolini O. Atala A, Cetrulo KJ, Taghizadeh RR, Murphy SV, Cetrulo CL, editors. 2018. Epithelial and mesenchymal stromal cells from the amniotic membrane. Elsevier Science & Technology;San Diego: p. 147–155. DOI: 10.1016/B978-0-12-812015-6.00011-X.7. Insausti CL, Blanquer M, García-Hernández AM, Castellanos G, Moraleda JM. 2014; Amniotic membrane-derived stem cells: immunomodulatory properties and potential clinical application. Stem Cells Cloning. 7:53–63. DOI: 10.2147/SCCAA.S58696. PMID: 24744610. PMCID: PMC3969346.
Article8. Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, Alizadeh H. 2005; Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 46:900–907. DOI: 10.1167/iovs.04-0495. PMID: 15728546.
Article9. Zhou S, Chen J, Feng J. 2003; The effects of amniotic membrane on polymorphonuclear cells. Chin Med J (Engl). 116:788–790. PMID: 12875703.10. Parolini O, Souza-Moreira L, O'Valle F, Magatti M, Hernandez-Cortes P, Gonzalez-Rey E, Delgado M. 2014; Therapeutic effect of human amniotic membrane-derived cells on experimental arthritis and other inflammatory disorders. Arthritis Rheumatol. 66:327–339. DOI: 10.1002/art.38206. PMID: 24504805.
Article11. Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, Benarafa C, Roos D, Skokowa J, Hartl D. 2015; Neutrophils: between host defence, immune modulation, and tissue injury. PLoS Pathog. 11:e1004651. DOI: 10.1371/journal.ppat.1004651. PMID: 25764063. PMCID: PMC4357453.
Article12. Nicolás-Ávila JÁ, Adrover JM, Hidalgo A. 2017; Neutrophils in homeostasis, immunity, and cancer. Immunity. 46:15–28. DOI: 10.1016/j.immuni.2016.12.012. PMID: 28099862.
Article13. Yang F, Feng C, Zhang X, Lu J, Zhao Y. 2017; The diverse biological functions of neutrophils, beyond the defense against infections. Inflammation. 40:311–323. DOI: 10.1007/s10753-016-0458-4. PMID: 27817110.
Article14. Liu W, Chen G. 2014; Cryopreservation of human pluripotent stem cells in defined medium. Curr Protoc Stem Cell Biol. 31:1C.17.1–1C.17.13. DOI: 10.1002/9780470151808.sc01c17s31. PMID: 25366897. PMCID: PMC4252050.
Article15. Motedayyen H, Esmaeil N, Tajik N, Khadem F, Ghotloo S, Khani B, Rezaei A. 2017; Method and key points for isolation of human amniotic epithelial cells with high yield, viability and purity. BMC Res Notes. 10:552. DOI: 10.1186/s13104-017-2880-6. PMID: 29096713. PMCID: PMC5669002.
Article16. Islam R, Rahman MS, Asaduzzaman SM, Rahman MS. 2015; Properties and therapeutic potential of human amniotic membrane. Asian J Dermatol. 7:1–12. DOI: 10.3923/ajd.2015.1.12.
Article17. Wolbank S, Peterbauer A, Fahrner M, Hennerbichler S, van Griensven M, Stadler G, Redl H, Gabriel C. 2007; Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 13:1173–1183. DOI: 10.1089/ten.2006.0313. PMID: 17518752.
Article18. Motedayyen H, Zarnani AH, Tajik N, Ghotloo S, Rezaei A. 2018; Immunomodulatory effects of human amniotic epithelial cells on naive CD4+ T cells from women with unexplained recurrent spontaneous abortion. Placenta. 71:31–40. DOI: 10.1016/j.placenta.2018.06.008. PMID: 30415745.
Article19. Banas RA, Trumpower C, Bentlejewski C, Marshall V, Sing G, Zeevi A. 2008; Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Hum Immunol. 69:321–328. DOI: 10.1016/j.humimm.2008.04.007. PMID: 18571002.
Article20. Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, Ottonello L, Pistoia V. 2008; Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 26:151–162. DOI: 10.1634/stemcells.2007-0416. PMID: 17932421.
Article21. Chen CP, Chen YY, Huang JP, Wu YH. 2014; The effect of conditioned medium derived from human placental multipotent mesenchymal stromal cells on neutrophils: possible implications for placental infection. Mol Hum Reprod. 20:1117–1125. DOI: 10.1093/molehr/gau062. PMID: 25140001.
Article22. Salami F, Tavassoli A, Mehrzad J, Parham A. 2018; Immunomodulatory effects of mesenchymal stem cells on leukocytes with emphasis on neutrophils. Immunobiology. 223:786–791. DOI: 10.1016/j.imbio.2018.08.002. PMID: 30119931.
Article23. Khan I, Zhang L, Mohammed M, Archer FE, Abukharmah J, Yuan Z, Rizvi SS, Melek MG, Rabson AB, Shi Y, Weinberger B, Vetrano AM. 2014; Effects of Wharton's jelly-derived mesenchymal stem cells on neonatal neutrophils. J Inflamm Res. 8:1–8. DOI: 10.2147/JIR.S71987. PMID: 25678809. PMCID: PMC4317142.24. Pourtayeb S, Abtahi Froushani SM. 2017; Nicotine can modulate the effects of the mesenchymal stem cells on neutrophils. Adv Med Sci. 62:165–170. DOI: 10.1016/j.advms.2016.07.006. PMID: 28282603.
Article25. Galeh HEG, Delirezh N, Abtahi Froushani SM, Afzale Ahangaran N. 2014; Calcitriol modulates the effects of the supernatants of bone-marrow-derived mesenchymal stem cells on neutrophil functions. Turk J Biol. 38:365–370. DOI: 10.3906/biy-1310-40.
Article26. Morandi F, Horenstein AL, Quarona V, Faini AC, Castella B, Srinivasan RC, Strom SC, Malavasi F, Gramignoli R. 2019; Ectonucleotidase expression on human amnion epithelial cells: adenosinergic pathways and dichotomic effects on immune effector cell populations. J Immunol. 202:724–735. DOI: 10.4049/jimmunol.1800432. PMID: 30587530.
Article27. Laranjeira P, Duque M, Vojtek M, Inácio MJ, Silva I, Mamede AC, Laranjo M, Pedreiro S, Carvalho MJ, Moura P, Abrantes AM, Maia CJ, Domingues P, Domingues R, Martinho A, Botelho MF, Trindade H, Paiva A. 2018; Amniotic membrane extract differentially regulates human peripheral blood T cell subsets, monocyte subpopulations and myeloid dendritic cells. Cell Tissue Res. 373:459–476. DOI: 10.1007/s00441-018-2822-1. PMID: 29582167.
Article28. Rossi D, Pianta S, Magatti M, Sedlmayr P, Parolini O. 2012; Characterization of the conditioned medium from amniotic membrane cells: prostaglandins as key effectors of its immunomodulatory activity. PLoS One. 7:e46956. DOI: 10.1371/journal.pone.0046956. PMID: 23071674. PMCID: PMC3468614.
Article29. Morandi F, Rizzo R, Fainardi E, Rouas-Freiss N, Pistoia V. 2016; Recent advances in our understanding of HLA-G biology: lessons from a wide spectrum of human diseases. J Immunol Res. 2016:4326495. DOI: 10.1155/2016/4326495. PMID: 27652273. PMCID: PMC5019910.
Article30. Sionov RV, Fridlender ZG, Granot Z. 2015; The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 8:125–158. DOI: 10.1007/s12307-014-0147-5. PMID: 24895166. PMCID: PMC4714999.
Article31. Shishikura K, Horiuchi T, Sakata N, Trinh DA, Shirakawa R, Kimura T, Asada Y, Horiuchi H. 2016; Prostaglandin E2 inhibits neutrophil extracellular trap formation through production of cyclic AMP. Br J Pharmacol. 173:319–331. DOI: 10.1111/bph.13373. PMID: 26505736. PMCID: PMC5341226.
Article32. Sabat R, Grütz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J. 2010; Biology of interleukin-10. Cytokine Growth Factor Rev. 21:331–344. DOI: 10.1016/j.cytogfr.2010.09.002. PMID: 21115385.
Article33. Loughman JA, Yarbrough ML, Tiemann KM, Hunstad DA. 2016; Local generation of kynurenines mediates inhibition of neutrophil chemotaxis by uropathogenic escherichia coli. Infect Immun. 84:1176–1183. DOI: 10.1128/IAI.01202-15. PMID: 26857571. PMCID: PMC4807489.
Article34. Bak DH, Na J, Choi MJ, Lee BC, Oh CT, Kim JY, Han HJ, Kim MJ, Kim TH, Kim BJ. 2018; Anti-apoptotic effects of human placental hydrolysate against hepatocyte toxicity in vivo and in vitro. Int J Mol Med. 42:2569–2583. DOI: 10.3892/ijmm.2018.3830. PMID: 30132515. PMCID: PMC6192762.35. Schumacher A, Sharkey DJ, Robertson SA, Zenclussen AC. 2018; Immune cells at the fetomaternal interface: how the microenvironment modulates immune cells to foster fetal development. J Immunol. 201:325–334. DOI: 10.4049/jimmunol.1800058. PMID: 29987001.
Article36. Gupta A, Kedige SD, Jain K. 2015; Amnion and chorion membranes: potential stem cell reservoir with wide applications in periodontics. Int J Biomater. 2015:274082. DOI: 10.1155/2015/274082. PMID: 26770199. PMCID: PMC4684856.
Article37. Gomez-Lopez N, Romero R, Xu Y, Miller D, Leng Y, Panaitescu B, Silva P, Faro J, Alhousseini A, Gill N, Hassan SS, Hsu CD. 2018; The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. Am J Reprod Immunol. 79:e12827. DOI: 10.1111/aji.12827. PMID: 29500850. PMCID: PMC5951617.
Article38. Zeng MY, Miralda I, Armstrong CL, Uriarte SM, Bagaitkar J. 2019; The roles of NADPH oxidase in modulating neutrophil effector responses. Mol Oral Microbiol. 34:27–38. DOI: 10.1111/omi.12252. PMID: 30632295. PMCID: PMC6935359.
Article39. Gomez-Lopez N, Romero R, Garcia-Flores V, Xu Y, Leng Y, Alhousseini A, Hassan SS, Panaitescu B. 2017; Amniotic fluid neutrophils can phagocytize bacteria: a mechanism for microbial killing in the amniotic cavity. Am J Reprod Immunol. 78:e12723. DOI: 10.1111/aji.12723. PMID: 28703488. PMCID: PMC5623137.
Article40. Gomez-Lopez N, Romero R, Xu Y, Leng Y, Garcia-Flores V, Miller D, Jacques SM, Hassan SS, Faro J, Alsamsam A, Alhousseini A, Gomez-Roberts H, Panaitescu B, Yeo L, Maymon E. 2017; Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol. 217:693.e1–693.e16. DOI: 10.1016/j.ajog.2017.09.013. PMID: 28964823. PMCID: PMC5878926.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term Outcome of Limbal Epithelial Cells Cultivated in Vivo on Amniotic Membrane Transplantation
- Conjunctival Epithelial Cells Auto-Cultivated In Vivo on Human Amniotic Membrane in Rabbits
- Transplantation of in vivo Cultivated Limbal Corneal Epithelial Cells with Total Limbal Stem Cell Deficiency
- Therapeutic Aspects of Mesenchymal Stem Cell-Based Cell Therapy with a Focus on Human Amniotic Epithelial Cells in Multiple Sclerosis: A Mechanistic Review
- Large-scale Isolation, Expansion and Characterization of Human Amniotic Epithelial Cells