Int J Stem Cells.
2021 Aug;14(3):241-251. 10.15283/ijsc21032.
Therapeutic Aspects of Mesenchymal Stem Cell-Based Cell Therapy with a Focus on Human Amniotic Epithelial Cells in Multiple Sclerosis: A Mechanistic Review
- Affiliations
-
- 1Department of Anatomy, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
- 2Autoimmune Diseases Research Center, Kashan University of Medical Sciences, Kashan, Iran
- 3Cell Biology and Molecular-Genetics Department, Marand Azad University, Marand, Iran
- KMID: 2519367
- DOI: http://doi.org/10.15283/ijsc21032
Abstract
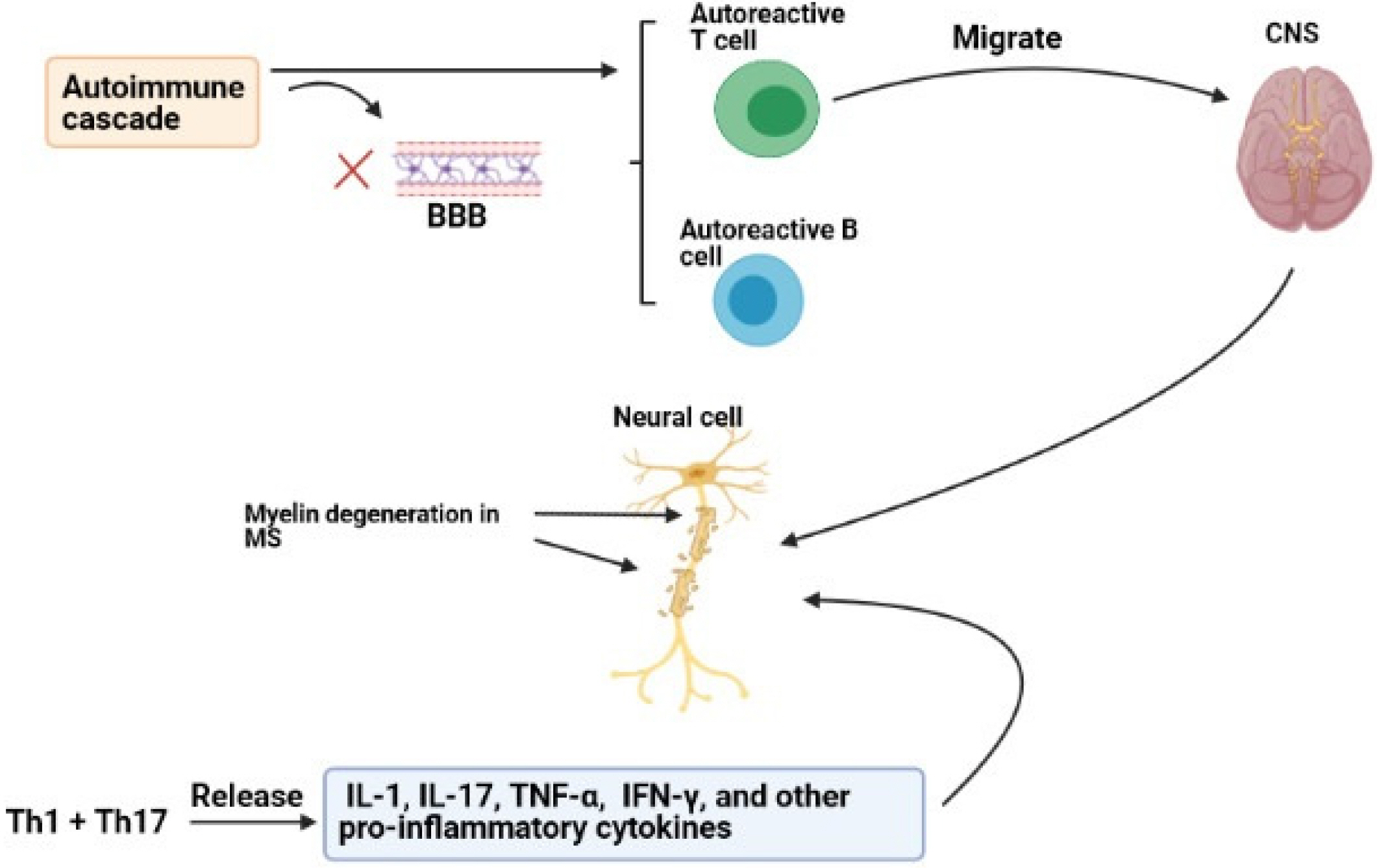
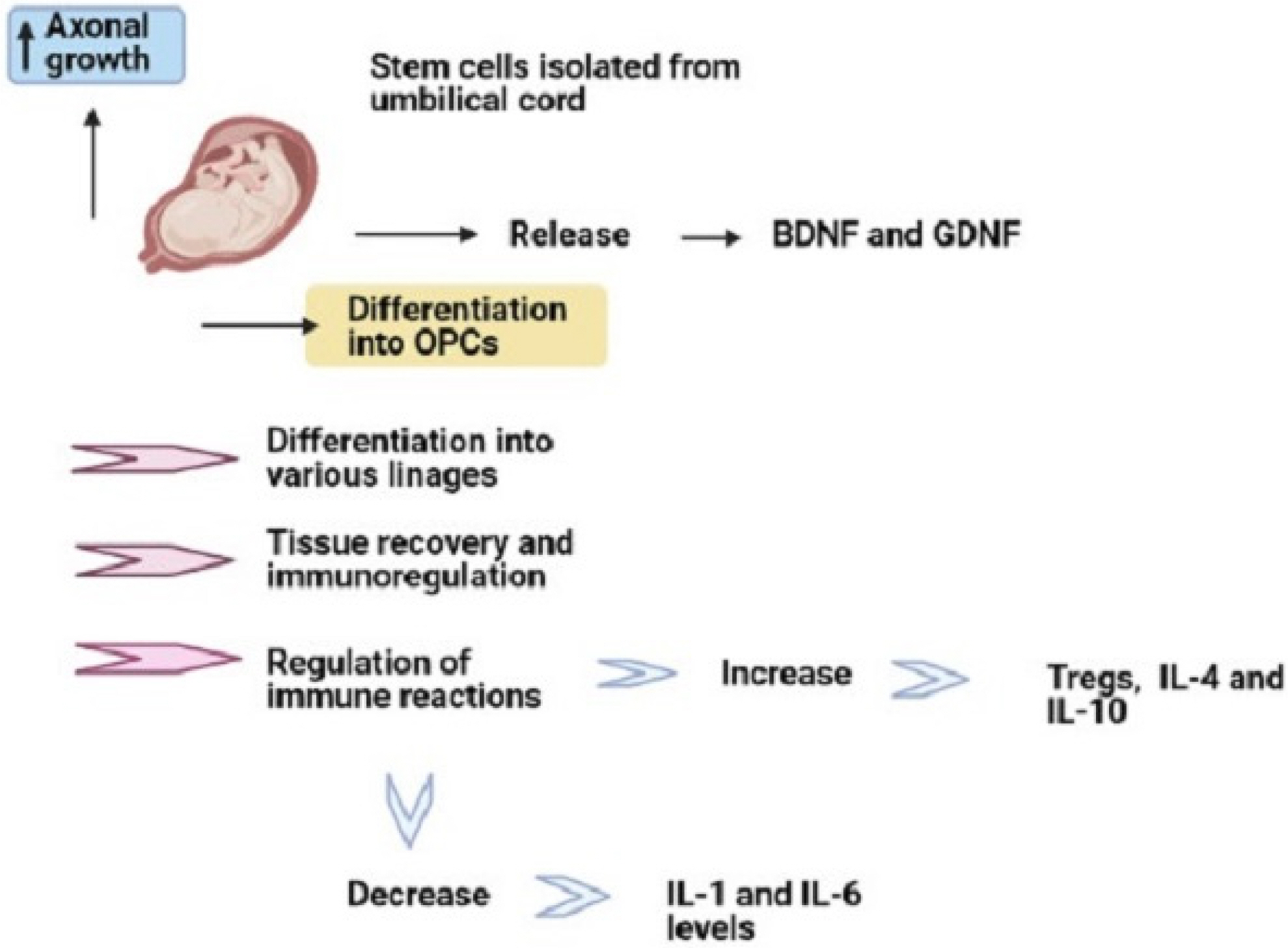
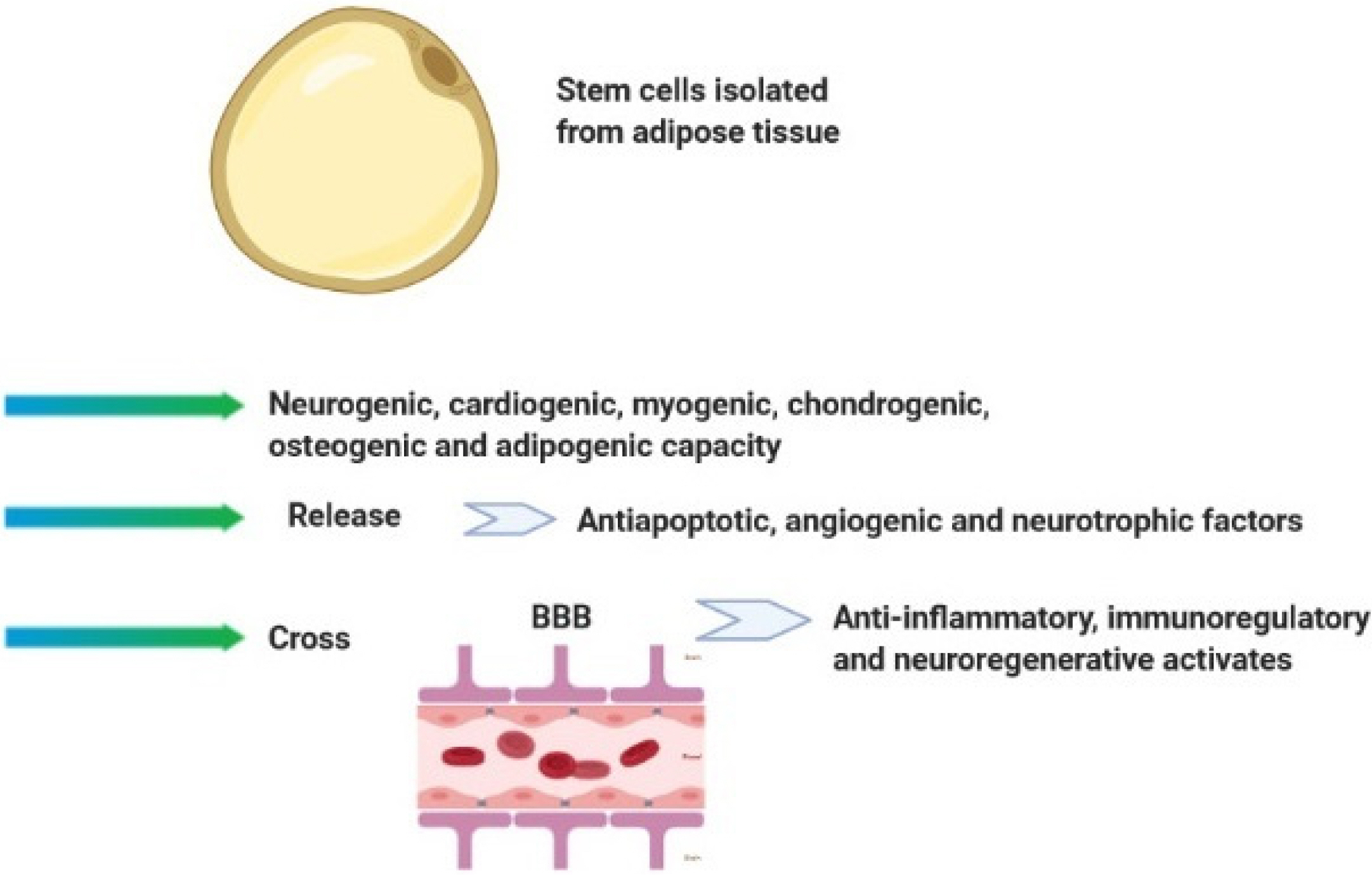
- Multiple sclerosis (MS) is an inflammatory disease of central nervous system (CNS). The mmune system plays an important role in its pathogenesis. Current treatments are unable to cure patients and prevent the progression of MS lesions. Stem cell-based cell therapy has opened a new window for MS treatment. Stem cells regulate immune responses and improve axonal remyelination. Stem cells can be obtained from different origins such as embryonic, neural, bone marrow, and adipose tissues. But yet there is a challenge for the selection of the best cell source for stem cell therapy. Mesenchymal stem cells (MSCs) are a type of stem cell obtained from different origins and have significant immunomodulatory effects on the immune system. The increasing evidence have suggested that umbilical cord and adipose tissue can be a suitable source for isolation of MSCs. Moreover, human amniotic epithelial cells (hAECs) as novel stem cell origins by having immunoregulatory effects, regenerative effects, and less capacity of antigenicity can be a candidate for MS treatment. This review discussed the mechanistic effects of MSCs with a focus on human amniotic epithelial cells, which can be used to treatment and improvement of outcome in MS disease.
Keyword
Figure
Reference
-
References
1. Martin JB. 1999; Molecular basis of the neurodegenerative disorders. N Engl J Med. 340:1970–1980. DOI: 10.1056/NEJM199906243402507. PMID: 10379022.
Article2. Jafarzadeh Bejargafshe M, Hedayati M, Zahabiasli S, Tahmasbpour E, Rahmanzadeh S, Nejad-Moghaddam A. 2019; Safety and efficacy of stem cell therapy for treatment of neural damage in patients with multiple sclerosis. Stem Cell Investig. 6:44. DOI: 10.21037/sci.2019.10.06. PMID: 32039266. PMCID: PMC6987330.
Article3. Minzenberg MJ. 2010; Dousing the flames to repair brains. Sci Transl Med. 2:27ec60. DOI: 10.1126/scitranslmed.3001146.
Article4. Yousefi F, Lavi Arab F, Saeidi K, Amiri H, Mahmoudi M. 2019; Various strategies to improve efficacy of stem cell transplantation in multiple sclerosis: focus on mesenchymal stem cells and neuroprotection. J Neuroimmunol. 328:20–34. DOI: 10.1016/j.jneuroim.2018.11.015. PMID: 30557687.
Article5. Ghasemi N. 2015; Therapeutic effects of adipose derived mesenchymal stem cells on remyelination process in inflammatory demyelinating diseases. J Histol Histopathol. 2:8. DOI: 10.7243/2055-091X-2-8.
Article6. Abdallah AN, Shamaa AA, El-Tookhy OS. 2019; Evaluation of treatment of experimentally induced canine model of multiple sclerosis using laser activated non-expanded adipose derived stem cells. Res Vet Sci. 125:71–81. DOI: 10.1016/j.rvsc.2019.05.016. PMID: 31152923.
Article7. Nguyen H, Zarriello S, Coats A, Nelson C, Kingsbury C, Gorsky A, Rajani M, Neal EG, Borlongan CV. 2019; Stem cell therapy for neurological disorders: a focus on aging. Neurobiol Dis. 126:85–104. DOI: 10.1016/j.nbd.2018.09.011. PMID: 30219376. PMCID: PMC6650276.
Article8. Namchaiw P, Wen H, Mayrhofer F, Chechneva O, Biswas S, Deng W. 2019; Temporal and partial inhibition of GLI1 in neural stem cells (NSCs) results in the early maturation of NSC derived oligodendrocytes in vitro. Stem Cell Res Ther. 10:272. DOI: 10.1186/s13287-019-1374-y. PMID: 31455382. PMCID: PMC6712625.
Article9. Genc B, Bozan HR, Genc S, Genc K. 2019; Stem cell therapy for multiple sclerosis. Adv Exp Med Biol. 1084:145–174. DOI: 10.1007/5584_2018_247. PMID: 30039439.
Article10. Bitsch A, Bruhn H, Vougioukas V, Stringaris A, Lassmann H, Frahm J, Brück W. 1999; Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am J Neuroradiol. 20:1619–1627. PMID: 10543631. PMCID: PMC7056180.11. Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. 1981; Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 2:1003–1005. DOI: 10.1016/S0140-6736(81)91212-5. PMID: 6118474.
Article12. Motedayyen H, Zarnani AH, Tajik N, Ghotloo S, Rezaei A. 2018; Immunomodulatory effects of human amniotic epithelial cells on naive CD4+ T cells from women with unexplained recurrent spontaneous abortion. Placenta. 71:31–40. DOI: 10.1016/j.placenta.2018.06.008. PMID: 30415745.
Article13. Darlington PJ, Boivin MN, Bar-Or A. 2011; Harnessing the therapeutic potential of mesenchymal stem cells in multiple sclerosis. Expert Rev Neurother. 11:1295–1303. DOI: 10.1586/ern.11.113. PMID: 21864075. PMCID: PMC3234364.
Article14. Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. 2005; Stem cell characteristics of amniotic epithelial cells. Stem Cells. 23:1549–1559. DOI: 10.1634/stemcells.2004-0357. PMID: 16081662.
Article15. Rahmati M, Ghannadian SM, Kasiri N, Ahmadi L, Motedayyen H, Shaygannejad V, Pourazar A, Alsahebfosoul F, Ganjalikhani Hakemi M, Eskandari N. 2021; Modulation of Th17 proliferation and IL-17A gene expression by acetylated form of Apigenin in patients with multiple sclerosis. Immunol Invest. 50:216–229. DOI: 10.1080/08820139.2020.1726381. PMID: 32100582.
Article16. Sedaghat N, Motedayyen H, Alsahebfosoul F, Etemadifar M, Ostadi V, Kianpour F, Zarkesh Esfahani SH. 2019; Increased expression of lymphocyte activation gene-3 by regulatory t cells in multiple sclerosis patients with fingolimod treatment Turkish J Immunol. 7:31–39. DOI: 10.25002/tji.2019.1035. PMID: 32100582.17. Imitola J. 2019; Regenerative neuroimmunology: the impact of immune and neural stem cell interactions for translation in neurodegeneration and repair. J Neuroimmunol. 331:1–3. DOI: 10.1016/j.jneuroim.2019.04.008. PMID: 31023492.
Article18. Motedayyen H, Rezaei A, Zarnani AH, Tajik N. 2018; Human amniotic epithelial cells inhibit activation and pro-inflammatory cytokines production of naive CD4+ T cells from women with unexplained recurrent spontaneous abortion. Reprod Biol. 18:182–188. DOI: 10.1016/j.repbio.2018.04.002. PMID: 29729842.
Article19. Sedaghat N, Motedayyen H, Etemadifar M, Zarkesh H, Kianpour F, Vestri E, Alsahebfosoul F. 2018; Effect of fingolimod on the frequency of regulatory T cells in patients with relapsing-remitting multiple sclerosis. J Immun Res. 5:1032.
Article20. Pirttilä T, Nurmikko T. 1995; CSF oligoclonal bands, MRI, and the diagnosis of multiple sclerosis. Acta Neurol Scand. 92:468–471. DOI: 10.1111/j.1600-0404.1995.tb00482.x. PMID: 8750112.
Article21. Markarian CF, Frey GZ, Silveira MD, Chem EM, Milani AR, Ely PB, Horn AP, Nardi NB, Camassola M. 2014; Isolation of adipose-derived stem cells: a comparison among different methods. Biotechnol Lett. 36:693–702. DOI: 10.1007/s10529-013-1425-x. PMID: 24322777.
Article22. Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. 2004; Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 22:649–658. DOI: 10.1634/stemcells.22-5-649. PMID: 15342929.
Article23. Schwab KE, Hutchinson P, Gargett CE. 2008; Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum Reprod. 23:934–943. DOI: 10.1093/humrep/den051. PMID: 18305000.
Article24. Xu C, Diao YF, Wang J, Liang J, Xu HH, Zhao ML, Zheng B, Luan Z, Wang JJ, Yang XP, Wei MG, Duan JH, Wang KQ, Chen C, Chen F, Ming D, Zhang S, Sun HT, Li XH. 2020; Intravenously infusing the secretome of adipose-derived mesenchymal stem cells ameliorates neuroinflammation and neurological functioning after traumatic brain injury. Stem Cells Dev. 29:222–234. DOI: 10.1089/scd.2019.0173. PMID: 31830866.
Article25. Song CG, Zhang YZ, Wu HN, Cao XL, Guo CJ, Li YQ, Zheng MH, Han H. 2018; Stem cells: a promising candidate to treat neurological disorders. Neural Regen Res. 13:1294–1304. DOI: 10.4103/1673-5374.235085. PMID: 30028342. PMCID: PMC6065243.
Article26. Samper Agrelo I, Schira-Heinen J, Beyer F, Groh J, Bütermann C, Estrada V, Poschmann G, Bribian A, Jadasz JJ, Lopez-Mascaraque L, Kremer D, Martini R, Müller HW, Hartung HP, Adjaye J, Stühler K, Küry P. 2020; Secretome analysis of mesenchymal stem cell factors fostering oligodendroglial differentiation of neural stem cells in vivo. Int J Mol Sci. 21:4350. DOI: 10.3390/ijms21124350. PMID: 32570968. PMCID: PMC7352621.
Article27. Jaramillo-Merchán J, Jones J, Ivorra JL, Pastor D, Viso-León MC, Armengól JA, Moltó MD, Geijo-Barrientos E, Martínez S. 2013; Mesenchymal stromal-cell transplants induce oligodendrocyte progenitor migration and remyelination in a chronic demyelination model. Cell Death Dis. 4:e779. DOI: 10.1038/cddis.2013.304. PMID: 23990019. PMCID: PMC3763464.
Article28. Forte A, Finicelli M, Mattia M, Berrino L, Rossi F, De Feo M, Cotrufo M, Cipollaro M, Cascino A, Galderisi U. 2008; Mesenchymal stem cells effectively reduce surgically induced stenosis in rat carotids. J Cell Physiol. 217:789–799. DOI: 10.1002/jcp.21559. PMID: 18690654.
Article29. Peng Z, Gao W, Yue B, Jiang J, Gu Y, Dai J, Chen L, Shi Q. 2018; Promotion of neurological recovery in rat spinal cord injury by mesenchymal stem cells loaded on nerve-guided collagen scaffold through increasing alternatively activated macrophage polarization. J Tissue Eng Regen Med. 12:e1725–e1736. DOI: 10.1002/term.2358. PMID: 27863083.
Article30. Brück W, Porada P, Poser S, Rieckmann P, Hanefeld F, Kretzschmar HA, Lassmann H. 1995; Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 38:788–796. DOI: 10.1002/ana.410380514. PMID: 7486871.
Article31. Freedman MS, Bar-Or A, Atkins HL, Karussis D, Frassoni F, Lazarus H, Scolding N, Slavin S, Le Blanc K, Uccelli A. 2010; The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: consensus report of the International MSCT Study Group. Mult Scler. 16:503–510. DOI: 10.1177/1352458509359727. PMID: 20086020.
Article32. Squillaro T, Peluso G, Galderisi U. 2016; Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 25:829–848. DOI: 10.3727/096368915X689622. PMID: 26423725.
Article33. Najar M, Fayyad-Kazan M, Meuleman N, Bron D, Fayyad-Kazan H, Lagneaux L. 2018; Mesenchymal stromal cells of the bone marrow and natural killer cells: cell interactions and cross modulation. J Cell Commun Signal. 12:673–688. DOI: 10.1007/s12079-018-0448-4. PMID: 29350342. PMCID: PMC6235772.
Article34. Liu X, Ren S, Qu X, Ge C, Cheng K, Zhao RC. 2015; Mesenchymal stem cells inhibit Th17 cells differentiation via IFN-γ-mediated SOCS3 activation. Immunol Res. 61:219–229. DOI: 10.1007/s12026-014-8612-2. PMID: 25588866.
Article35. Wang D, Huang S, Yuan X, Liang J, Xu R, Yao G, Feng X, Sun L. 2017; The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythema-tosus. Cell Mol Immunol. 14:423–431. DOI: 10.1038/cmi.2015.89. PMID: 26435067. PMCID: PMC5423084.
Article36. Selmani Z, Naji A, Gaiffe E, Obert L, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. 2009; HLA-G is a crucial immunosuppressive molecule secreted by adult human mesenchymal stem cells. Transplantation. 87(9 Suppl):S62–S66. DOI: 10.1097/TP.0b013e3181a2a4b3. PMID: 19424010.
Article37. Lu Z, Chang W, Meng S, Xu X, Xie J, Guo F, Yang Y, Qiu H, Liu L. 2019; Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury. Stem Cell Res Ther. 10:372. DOI: 10.1186/s13287-019-1488-2. PMID: 31801626. PMCID: PMC6894226.
Article38. Razmkhah M, Jaberipour M, Erfani N, Habibagahi M, Talei AR, Ghaderi A. 2011; Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-β1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cell Immunol. 266:116–122. DOI: 10.1016/j.cellimm.2010.09.005. PMID: 20970781.
Article39. Duffy MM, Ritter T, Ceredig R, Griffin MD. 2011; Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther. 2:34. DOI: 10.1186/scrt75. PMID: 21861858. PMCID: PMC3219065.
Article40. Uccelli A, Laroni A, Freedman MS. 2011; Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 10:649–656. DOI: 10.1016/S1474-4422(11)70121-1. PMID: 21683930.
Article41. Ryu CH, Park KY, Hou Y, Jeong CH, Kim SM, Jeun SS. 2013; Gene therapy of multiple sclerosis using interferon β-se-creting human bone marrow mesenchymal stem cells. Biomed Res Int. 2013:696738. DOI: 10.1155/2013/696738. PMID: 23710456. PMCID: PMC3654641.42. Patel SA, Sherman L, Munoz J, Rameshwar P. 2008; Immu-nological properties of mesenchymal stem cells and clinical implications. Arch Immunol Ther Exp (Warsz). 56:1–8. DOI: 10.1007/s00005-008-0001-x. PMID: 18250975.
Article43. Yamout B, Hourani R, Salti H, Barada W, El-Hajj T, Al-Kutoubi A, Herlopian A, Baz EK, Mahfouz R, Khalil-Hamdan R, Kreidieh NM, El-Sabban M, Bazarbachi A. 2010; Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroi-mmunol. 227:185–189. DOI: 10.1016/j.jneuroim.2010.07.013. PMID: 20728948.
Article44. Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers GL. 2002; Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 30:783–791. DOI: 10.1016/S0301-472X(02)00812-3. PMID: 12135677.
Article45. Ding Y, Yang H, Feng JB, Qiu Y, Li DS, Zeng Y. 2013; Human umbilical cord-derived MSC culture: the replacement of animal sera with human cord blood plasma. In Vitro Cell Dev Biol Anim. 49:771–777. DOI: 10.1007/s11626-013-9663-8. PMID: 24043577.
Article46. Hu J, Yu X, Wang Z, Wang F, Wang L, Gao H, Chen Y, Zhao W, Jia Z, Yan S, Wang Y. 2013; Long term effects of the implantation of Wharton's jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr J. 60:347–357. DOI: 10.1507/endocrj.EJ12-0343. PMID: 23154532.
Article47. Li JF, Yin HL, Shuboy A, Duan HF, Lou JY, Li J, Wang HW, Wang YL. 2013; Differentiation of hUC-MSC into dopaminergic-like cells after transduction with hepatocyte growth factor. Mol Cell Biochem. 381:183–190. DOI: 10.1007/s11010-013-1701-z. PMID: 23737134.
Article48. Liu R, Zhang Z, Lu Z, Borlongan C, Pan J, Chen J, Qian L, Liu Z, Zhu L, Zhang J, Xu Y. 2013; Human umbilical cord stem cells ameliorate experimental autoimmune encephalomyelitis by regulating immunoinflammation and remye-lination. Stem Cells Dev. 22:1053–1062. DOI: 10.1089/scd.2012.0463. PMID: 23140594.49. Rong LJ, Chi Y, Yang SG, Chen DD, Chen F, Xu SX, Zhang DL, Ma FX, Lu SH, Han ZC. 2012; Effects of interferon-γ on biological characteristics and immunomodulatory property of human umbilical cord-derived mesenchymal stem cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 20:421–426. Chinese. PMID: 22541112.50. Lu Z, Zhao H, Xu J, Zhang Z, Zhang X, Zhang Y, Liu Z, Xu Y. 2013; Human umbilical cord mesenchymal stem cells in the treatment of secondary progressive multiple sclerosis. J Stem Cell Res Ther. S6:2.
Article51. Yang H, Yang H, Xie Z, Wei L, Bi J. 2013; Systemic transplantation of human umbilical cord derived mesenchymal stem cells-educated T regulatory cells improved the impaired cognition in AβPPswe/PS1dE9 transgenic mice. PLoS One. 8:e69129. DOI: 10.1371/journal.pone.0069129. PMID: 23935936. PMCID: PMC3723739.
Article52. Dong H, Li G, Shang C, Yin H, Luo Y, Meng H, Li X, Wang Y, Lin L, Zhao M. 2018; Umbilical cord mesenchymal stem cell (UC-MSC) transplantations for cerebral palsy. Am J Transl Res. 10:901–906. PMID: 29636880. PMCID: PMC5883131.53. Yang H, Sun J, Wang F, Li Y, Bi J, Qu T. 2016; Umbilical cord-derived mesenchymal stem cells reversed the suppressive deficiency of T regulatory cells from peripheral blood of patients with multiple sclerosis in a co-culture-a preliminary study. Oncotarget. 7:72537–72545. DOI: 10.18632/oncotarget.12345. PMID: 27705922. PMCID: PMC5341927.54. Mazini L, Rochette L, Amine M, Malka G. 2019; Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int J Mol Sci. 20:2523. DOI: 10.3390/ijms20102523. PMID: 31121953. PMCID: PMC6566837.
Article55. Ragerdi Kashani I, Hedayatpour A, Pasbakhsh P, Kafami L, Atlasi N, Pirhajati Mahabadi V, Mamoudi R, Baazm M. 2012; 17β-estradiol enhances the efficacy of adipose-derived mesenchymal stem cells on remyelination in mouse model of multiple sclerosis. Acta Med Iran. 50:789–797. PMID: 23456519.56. Stepien A, Dabrowska NL, Maciagowska M, Macoch RP, Zolocinska A, Mazur S, Siennicka K, Frankowska E, Kidzinski R, Chalimoniuk M, Pojda Z. 2016; Clinical application of autologous adipose stem cells in patients with multiple sclerosis: preliminary results. Mediators Inflamm. 2016:5302120. DOI: 10.1155/2016/5302120. PMID: 27761060. PMCID: PMC5059576.
Article57. Gadelkarim M, Abushouk AI, Ghanem E, Hamaad AM, Saad AM, Abdel-Daim MM. 2018; Adipose-derived stem cells: effectiveness and advances in delivery in diabetic wound healing. Biomed Pharmacother. 107:625–633. DOI: 10.1016/j.biopha.2018.08.013. PMID: 30118878.
Article58. Chi K, Fu RH, Huang YC, Chen SY, Hsu CJ, Lin SZ, Tu CT, Chang LH, Wu PA, Liu SP. 2018; Adipose-derived stem cells stimulated with n-butylidenephthalide exhibit therapeutic effects in a mouse model of Parkinson's disease. Cell Transplant. 27:456–470. DOI: 10.1177/0963689718757408. PMID: 29756519. PMCID: PMC6038049.
Article59. Li J, Chen Y, Chen Z, Huang Y, Yang D, Su Z, Weng Y, Li X, Zhang X. 2017; Therapeutic effects of human adipose tissue-derived stem cell (hADSC) transplantation on experi-mental autoimmune encephalomyelitis (EAE) mice. Sci Rep. 7:42695. DOI: 10.1038/srep42695. PMID: 28198408. PMCID: PMC5309875.
Article60. Dai R, Wang Z, Samanipour R, Koo KI, Kim K. 2016; Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016:6737345. DOI: 10.1155/2016/6737345. PMID: 27057174. PMCID: PMC4761677.
Article61. Ghasemi N, Razavi S, Mardani M, Esfandiari E, Salehi H, Zarkesh Esfahani SH. 2014; Transplantation of human adipose-derived stem cells enhances remyelination in lysolecithin-induced focal demyelination of rat spinal cord. Mol Biotechnol. 56:470–478. DOI: 10.1007/s12033-014-9744-2. PMID: 24570177.
Article62. Tomita K, Madura T, Mantovani C, Terenghi G. 2012; Differentiated adipose-derived stem cells promote myelination and enhance functional recovery in a rat model of chronic denervation. J Neurosci Res. 90:1392–1402. DOI: 10.1002/jnr.23002. PMID: 22419645.
Article63. Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, Alizadeh H. 2005; Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Oph-thalmol Vis Sci. 46:900–907. DOI: 10.1167/iovs.04-0495. PMID: 15728546.
Article64. Insausti CL, Blanquer M, García-Hernández AM, Castellanos G, Moraleda JM. 2014; Amniotic membrane-derived stem cells: immunomodulatory properties and potential clinical appli-cation. Stem Cells Cloning. 7:53–63. DOI: 10.2147/SCCAA.S58696. PMID: 24744610. PMCID: PMC3969346.
Article65. Fathi F, Atapour A, Eskandari N, Keyhanmehr N, Hafezi H, Mohammadi S, Motedayyen H. 2019; Regulatory T-cells and their impacts on cytokine profile of end-stage renal disease patients suffering from systemic lupus erythematosus. Int J Immunopathol Pharmacol. 33:2058738419863238. DOI: 10.1177/2058738419863238. PMID: 31280608. PMCID: PMC6614948.
Article66. Yang PJ, Yuan WX, Liu J, Li JY, Tan B, Qiu C, Zhu XL, Qiu C, Lai DM, Guo LH, Yu LY. 2018; Biological characterization of human amniotic epithelial cells in a serum-free system and their safety evaluation. Acta Pharmacol Sin. 39:1305–1316. DOI: 10.1038/aps.2018.22. PMID: 29565036. PMCID: PMC6289351.
Article67. McDonald CA, Short M, Jenkin G, Bernard CCA. Atala A, Murphy S, editors. 2014. The potential of human amnion epithelial cells as an immuno-modulatory and neuroregenerative treatment for multiple sclerosis. Perinatal Stem Cells. Springer;New York: p. 231–242. DOI: 10.1007/978-1-4939-1118-9_21.
Article68. Alipour R, Motedayyen H, Sereshki N, Rafiee M, Alsahebfosul F, Pourazar A. 2020; Human amniotic epithelial cells affect the functions of neutrophils. Int J Stem Cells. 13:212–220. DOI: 10.15283/ijsc19155. PMID: 32323513. PMCID: PMC7378904.
Article69. Khadem F, Esmaeil N, Rezaei A, Motadayen H, Khani B. 2019; Immunoregulatory effects of human amnion epithelial cells on natural killer and T cells in women with Recurrent Spontaneous Abortion (RSA). Turk J Immunol. 7:21–30. DOI: 10.25002/tji.2019.991.
Article70. McDonald CA, Payne NL, Sun G, Moussa L, Siatskas C, Lim R, Wallace EM, Jenkin G, Bernard CC. 2015; Immuno-suppressive potential of human amnion epithelial cells in the treatment of experimental autoimmune encephalomyelitis. J Neuroinflammation. 12:112. DOI: 10.1186/s12974-015-0322-8. PMID: 26036872. PMCID: PMC4457975.
Article71. Motedayyen H, Esmaeil N, Tajik N, Khadem F, Ghotloo S, Khani B, Rezaei A. 2017; Method and key points for isolation of human amniotic epithelial cells with high yield, viability and purity. BMC Res Notes. 10:552. DOI: 10.1186/s13104-017-2880-6. PMID: 29096713. PMCID: PMC5669002.
Article72. Taheri RA, Motedayyen H, Ghotloo S, Masjedi M, Mosaffa N, Mirshafiey A, Saffari M. 2018; The effect of lipopolysaccha-ride on the expression level of immunomodulatory and immunostimulatory factors of human amniotic epithelial cells. BMC Res Notes. 11:343. DOI: 10.1186/s13104-018-3411-9. PMID: 29843819. PMCID: PMC5975661.
Article73. Motedayyen H, Fathi F, Fasihi-Ramandi M, Ali Taheri R. 2018; The effect of lipopolysaccharide on anti-inflammatory and pro-inflammatory cytokines production of human amniotic epithelial cells. Reprod Biol. 18:404–409. DOI: 10.1016/j.repbio.2018.09.005. PMID: 30220549.
Article74. Liu YH, Vaghjiani V, Tee JY, To K, Cui P, Oh DY, Manuelpillai U, Toh BH, Chan J. 2012; Amniotic epithelial cells from the human placenta potently suppress a mouse model of multiple sclerosis. PLoS One. 7:e35758. DOI: 10.1371/journal.pone.0035758. PMID: 22563398. PMCID: PMC3338525.
Article75. Xu H, Zhang J, Tsang KS, Yang H, Gao WQ. 2019; Therapeutic potential of human amniotic epithelial cells on injuries and disorders in the central nervous system. Stem Cells Int. 2019:5432301. DOI: 10.1155/2019/5432301. PMID: 31827529. PMCID: PMC6886344.
Article76. Han K, Lee JE, Kwon SJ, Park SY, Shim SH, Kim H, Moon JH, Suh CS, Lim HJ. 2008; Human amnion-derived mesenchymal stem cells are a potential source for uterine stem cell therapy. Cell Prolif. 41:709–725. DOI: 10.1111/j.1365-2184.2008.00553.x. PMID: 18823496. PMCID: PMC6496450.
Article77. Irony-Tur-Sinai M, Grigoriadis N, Tsiantoulas D, Touloumi O, Abramsky O, Brenner T. 2009; Immunomodulation of EAE by alpha-fetoprotein involves elevation of immune cell apoptosis markers and the transcription factor FoxP3. J Neurol Sci. 279:80–87. DOI: 10.1016/j.jns.2008.12.014. PMID: 19171355.
Article78. Yawno T, Schuilwerve J, Moss TJ, Vosdoganes P, Westover AJ, Afandi E, Jenkin G, Wallace EM, Miller SL. 2013; Human amnion epithelial cells reduce fetal brain injury in response to intrauterine inflammation. Dev Neurosci. 35:272–282. DOI: 10.1159/000346683. PMID: 23571644.
Article79. Meng XT, Chen D, Dong ZY, Liu JM. 2007; Enhanced neural differentiation of neural stem cells and neurite growth by amniotic epithelial cell co-culture. Cell Biol Int. 31:691–698. DOI: 10.1016/j.cellbi.2006.11.038. PMID: 17336104.
Article80. Uchida S, Suzuki Y, Araie M, Kashiwagi K, Otori Y, Sakuragawa N. 2003; Factors secreted by human amniotic epithelial cells promote the survival of rat retinal ganglion cells. Neurosci Lett. 341:1–4. DOI: 10.1016/S0304-3940(02)01454-4. PMID: 12676329.
Article81. Uchida S, Inanaga Y, Kobayashi M, Hurukawa S, Araie M, Sakuragawa N. 2000; Neurotrophic function of conditioned medium from human amniotic epithelial cells. J Neurosci Res. 62:585–590. DOI: 10.1002/1097-4547(20001115)62:4<585::AID-JNR13>3.0.CO;2-U. PMID: 11070502.
Article82. Kim EY, Lee KB, Kim MK. 2014; The potential of mesenchymal stem cells derived from amniotic membrane and amniotic fluid for neuronal regenerative therapy. BMB Rep. 47:135–140. DOI: 10.5483/BMBRep.2014.47.3.289. PMID: 24499672. PMCID: PMC4163884.
Article83. Wu Q, Fang T, Lang H, Chen M, Shi P, Pang X, Qi G. 2017; Comparison of the proliferation, migration and angiogenic properties of human amniotic epithelial and mesenchymal stem cells and their effects on endothelial cells. Int J Mol Med. 39:918–926. DOI: 10.3892/ijmm.2017.2897. PMID: 28259958. PMCID: PMC5360425.
Article84. Motamedi B, Ibrahim TAT, Abdul AB, Allaudin ZN, Moshrefi M, Hajghani M, Nematollahi-Mahani SN. 2015; Characteristics of human amniotic epithelial cells and bone marrow mesenchymal stem cells in a parallel study: hanging drop colony formation and doubling time. J Regen Med. 4:1.
Article85. Wu KC, Chang YH, Liu HW, Ding DC. 2019; Transplanting human umbilical cord mesenchymal stem cells and hyaluronate hydrogel repairs cartilage of osteoarthritis in the minipig model. Ci Ji Yi Xue Za Zhi. 31:11–19. DOI: 10.4103/tcmj.tcmj_87_18. PMID: 30692826. PMCID: PMC6334562.
Article86. Debnath T, Chelluri LK. 2019; Standardization and quality assessment for clinical grade mesenchymal stem cells from human adipose tissue. Hematol Transfus Cell Ther. 41:7–16. DOI: 10.1016/j.htct.2018.05.001. PMID: 30793099. PMCID: PMC6371406.
Article87. Serras ASM, Cipriano MZdRF, da Graça Silva PM, Miranda JPG. Kitala D, Maurício AC, editors. 2021. Challenges for deriving hepatocyte-like cells from umbilical cord mesenchymal stem cells for in vitro toxicology applications. Novel Perspectives of Stem Cell Manufacturing and Therapies. IntechOpen;London: p. 1–28.88. Xu Z, Wei W, Gagneur J, Perocchi F, Clauder-Münster S, Camblong J, Guffanti E, Stutz F, Huber W, Steinmetz LM. 2009; Bidirectional promoters generate pervasive transcription in yeast. Nature. 457:1033–1037. DOI: 10.1038/nature07728. PMID: 19169243. PMCID: PMC2766638.
Article89. Kachgal S, Putnam AJ. 2011; Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis. 14:47–59. DOI: 10.1007/s10456-010-9194-9. PMID: 21104120. PMCID: PMC3369878.
Article90. Si J, Dai J, Zhang J, Liu S, Gu J, Shi J, Shen SG, Guo L. 2015; Comparative investigation of human amniotic epithelial cells and mesenchymal stem cells for application in bone tissue engineering. Stem Cells Int. 2015:565732. DOI: 10.1155/2015/565732. PMID: 25834575. PMCID: PMC4365333.
Article91. Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Redl H, Sakuragawa N, Wolbank S, Zeisberger S, Zisch A, Strom SC. 2008; Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 26:300–311. DOI: 10.1634/stemcells.2007-0594. PMID: 17975221.
Article92. Banas RA, Trumpower C, Bentlejewski C, Marshall V, Sing G, Zeevi A. 2008; Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Hum Immunol. 69:321–328. DOI: 10.1016/j.humimm.2008.04.007. PMID: 18571002.
Article93. Mohyeddin Bonab M, Yazdanbakhsh S, Lotfi J, Alimoghaddom K, Talebian F, Hooshmand F, Ghavamzadeh A, Nikbin B. 2007; Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran J Immunol. 4:50–57. PMID: 17652844.94. Fernández O, Izquierdo G, Fernández V, Leyva L, Reyes V, Guerrero M, León A, Arnaiz C, Navarro G, Páramo MD, Cuesta A, Soria B, Hmadcha A, Pozo D, Fernandez-Montesinos R, Leal M, Ochotorena I, Gálvez P, Geniz MA, Barón FJ, Mata R, Medina C, Caparrós-Escudero C, Cardesa A, Cuende N. 2018; Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: a triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS One. 13:e0195891. DOI: 10.1371/journal.pone.0195891. PMID: 29768414. PMCID: PMC5955528.
Article95. Staff NP, Madigan NN, Morris J, Jentoft M, Sorenson EJ, Butler G, Gastineau D, Dietz A, Windebank AJ. 2016; Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS. Neurology. 87:2230–2234. DOI: 10.1212/WNL.0000000000003359. PMID: 27784774. PMCID: PMC5123559.
Article96. Li JF, Zhang DJ, Geng T, Chen L, Huang H, Yin HL, Zhang YZ, Lou JY, Cao B, Wang YL. 2014; The potential of human umbilical cord-derived mesenchymal stem cells as a novel cellular therapy for multiple sclerosis. Cell Transplant. 23 Suppl 1:S113–S122. DOI: 10.3727/096368914X685005. PMID: 25385295.
Article97. Riordan NH, Morales I, Fernández G, Allen N, Fearnot NE, Leckrone ME, Markovich DJ, Mansfield D, Avila D, Patel AN, Kesari S, Paz Rodriguez J. 2018; Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. J Transl Med. 16:57. DOI: 10.1186/s12967-018-1433-7. PMID: 29523171. PMCID: PMC5845260.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem Cell Therapy for Neurodegenerative Diseases
- Clinical utilization of cord blood over human health: experience of stem cell transplantation and cell therapy using cord blood in Korea
- Current Trends and Prospect of Cell Therapy using Hematopoietic Stem Cells
- The hope and hype of stem cell therapy
- Current Concepts of Stem Cell Therapy