Yonsei Med J.
2019 Jan;60(1):79-87. 10.3349/ymj.2019.60.1.79.
Matrine Suppresses Pancreatic Fibrosis by Regulating TGF-β/Smad Signaling in Rats
- Affiliations
-
- 1Department of Gastroenterology, the First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China. liupi126@sina.com
- 2Department of Gastroenterology, Nanchang University, Nanchang, Jiangxi, China.
- 3Department of Gastroenterology, Chinese People's Liberation Army No.171 Hospital, Jiujiang, Jiangxi, China.
- KMID: 2428281
- DOI: http://doi.org/10.3349/ymj.2019.60.1.79
Abstract
- PURPOSE
This study aimed to elucidate the molecular mechanisms of the anti-pancreatic fibrosis effects of matrine in rats.
MATERIALS AND METHODS
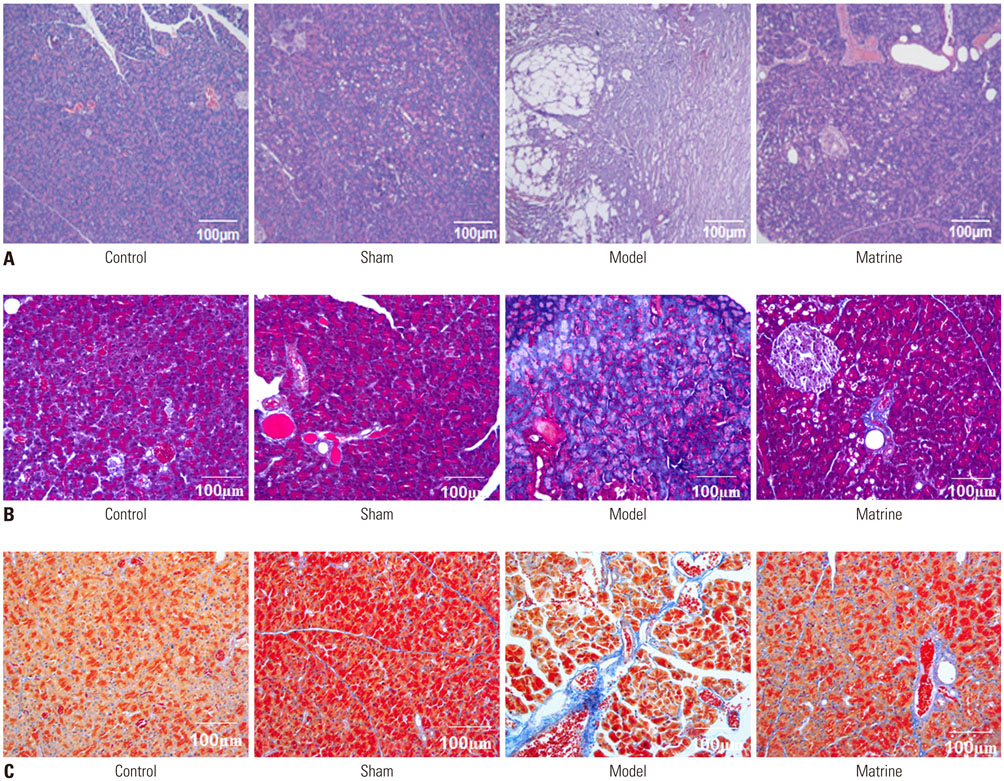
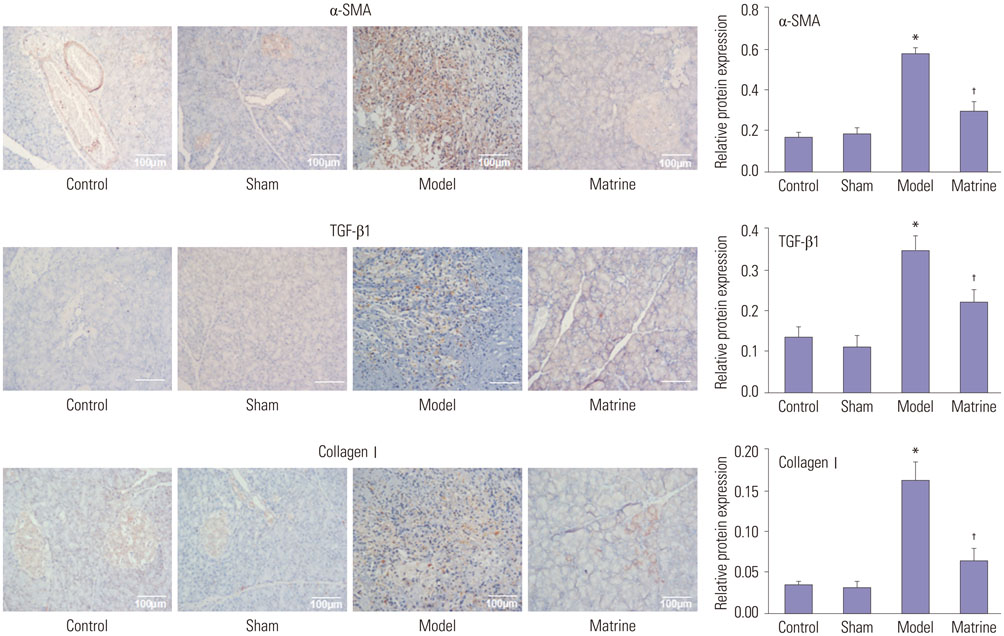
Trinitrobenzene sulfonic acid was administrated to rats to establish a pancreatic fibrosis model. Rats were divided into four groups: Control, Sham, Model, and Matrine (n=8). Hematoxylin-eosin staining, Masson staining, and Azan staining were performed to evaluate pancreatic fibrosis. Expression of transforming growth factor-β1 (TGF-β1), α-smooth muscle actin (α-SMA), and collagen I in pancreatic tissues was evaluated by immunohistochemical staining. mRNA and protein levels of TGF-β receptor 1 (TβR1), TβR2, and Smad2 in pancreatic tissues were determined by RT-PCR and Western blot, respectively.
RESULTS
In the model group, hyperplasia of glandules around the glandular ducts, mitochondrial swelling of acinous cells, and severe fibrosis were found. Interestingly, in the Matrine group, mitochondrial swelling was only found in a small number of acinous cells, and the fundamental structures of pancreatic tissues were intact. Moreover, pancreatic fibrosis was markedly alleviated. Comparing to the Sham group, expression of α-SMA, TGF-β1, and collagen I was sharply elevated in the Model group (p < 0.05); however, their expressions were much lower in the Matrine group, compared to the Model group (p < 0.05). Compared with the Sham group, mRNA and protein levels of Smad2, TβR1, and TβR2 in the Model group were notably raised (p < 0.05). However, their high expression was significantly downregulated in the Matrine group (p < 0.05).
CONCLUSION
Matrine suppressed pancreatic fibrosis by regulating TGF-β/Smad signaling in rats.
Keyword
MeSH Terms
Figure
Reference
-
1. Xue J, Sharma V, Hsieh MH, Chawla A, Murali R, Pandol SJ, et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun. 2015; 6:7158.
Article2. Tanaka K, Tomita H, Osada S, Watanabe H, Imai H, Sasaki Y, et al. Significance of histopathological evaluation of pancreatic fibrosis to predict postoperative course after pancreatic surgery. Anticancer Res. 2015; 35:1749–1756.3. Lu X, Chen Y. Pathologic assessment of pancreatic fibrosis in predicting pancreatic fistula and management of prophylactic drain removal after pancreaticoduodenectomy. World J Surg. 2016; 40:1520–1521.
Article4. Nagathihalli NS, Castellanos JA, VanSaun MN, Dai X, Ambrose M, Guo Q, et al. Pancreatic stellate cell secreted IL-6 stimulates STAT3 dependent invasiveness of pancreatic intraepithelial neoplasia and cancer cells. Oncotarget. 2016; 7:65982–65992.
Article5. Tjomsland V, Sandnes D, Pomianowska E, Cizmovic ST, Aasrum M, Brusevold IJ, et al. The TGFβ-SMAD3 pathway inhibits IL-1α induced interactions between human pancreatic stellate cells and pancreatic carcinoma cells and restricts cancer cell migration. J Exp Clin Cancer Res. 2016; 35:122.
Article6. Tjomsland V, Pomianowska E, Aasrum M, Sandnes D, Verbeke CS, Gladhaug IP. Profile of MMP and TIMP expression in human pancreatic stellate cells: regulation by IL-1α and TGFβ and implications for migration of pancreatic cancer cells. Neoplasia. 2016; 18:447–456.
Article7. Xu L, Zheng N, He Q, Li R, Zhang K, Liang T. Puerarin, isolated from Pueraria lobata (Willd.), protects against hepatotoxicity via specific inhibition of the TGF-β1/Smad signaling pathway, thereby leading to anti-fibrotic effect. Phytomedicine. 2013; 20:1172–1179.
Article8. Zhang YJ, Chan J, Xiang MX, Cheng G, Wang SS. Effect of matrine on fibrosis of hypertrophy myocardium induced by pressure overload. Bull Sci Technol. 2007; 23:67–71.9. Gao HY, Li GY, Lou MM, Li XY, Wei XY, Wang JH. Hepatoprotective effect of Matrine salvianolic acid B salt on carbon tetrachloride-induced hepatic fibrosis. J Inflamm (Lond). 2012; 9:16.
Article10. Zhang HW, Jin Y. [Renoprotective effects of matrine on experimental glomerulosclerosis in rats]. Zhonghua Er Ke Za Zhi. 2004; 42:737–740.11. Yuan X, Sun H, Miao R, Zhang Z, Zhao X, Guo Y, et al. Effect of matrine on NF-κB and collagen protein III of rats with pulmonary fibrosis. J Xinxiang Med College. 2009; 26:327–330.12. Bikádi P, Szabó J, Szabára Á, Jakab C. Internal positive controls of alpha-smooth muscle actin (α-SMA) in bovine tissues Immunohistochemical study. Magyar Allatorvosok Lapja. 2015; 137:151–158.13. Meng LP, Wang GG, Hu MX, Zhang H, Guan B, Hong YP. The correlation of magnetic resonance perfusion parameters and CD34, a-SMA of liver fibrosis and cirrhosis in rats. Radiol Pract. 2016; 31:580–585.14. Hu GX, Wan ZY, Shao JL, Zhang Y, Zhang LL, Gong ZJ. [Effects of hydroxycamptothecin on TGFb1, a-SMA and collagen I expression in rat hepatic satellite cells]. Zhonghua Gan Zang Bing Za Zhi. 2012; 20:453–457.15. De Jesus Araújo L, Yamamoto De Almeida L, Santos Lima J, Martelli-Júnior H, Ferreti Bonan PR. Evaluation of MMP-1, MMP-10, TIMP-1, a-SMA and TGF-b1 in angiofibromas of tuberous sclerosis. Minerva Stomatol. 2011; 60:25–33.16. David CJ, Huang YH, Chen M, Su J, Zou Y, Bardeesy N, et al. TGF-β tumor suppression through a lethal EMT. Cell. 2016; 164:1015–1030.
Article17. Zhang H, Kozono DE, O'Connor KW, Vidal-Cardenas S, Rousseau A, Hamilton A, et al. TGF-β inhibition rescues hematopoietic stem cell defects and bone marrow failure in Fanconi anemia. Cell Stem Cell. 2016; 18:668–681.
Article18. Yan X, Liao H, Cheng M, Shi X, Lin X, Feng XH, et al. Smad7 protein interacts with receptor-regulated Smads (R-Smads) to inhibit transforming growth factor-β (TGF-β)/Smad signaling. J Biol Chem. 2016; 291:382–392.
Article19. Da C, Liu Y, Zhan Y, Liu K, Wang R. Nobiletin inhibits epithelialmesenchymal transition of human non-small cell lung cancer cells by antagonizing the TGF-β1/Smad3 signaling pathway. Oncol Rep. 2016; 35:2767–2774.
Article20. Ma M, He M, Jiang Q, Yan Y, Guan S, Zhang J, et al. MiR-487a promotes TGF-β1-induced EMT, the migration and invasion of breast cancer cells by directly targeting MAGI2. Int J Biol Sci. 2016; 12:397–408.
Article21. Zhang YB, Zhan LQ, Li GQ, Wang F, Wang Y, Li YL, et al. Dimeric matrine-type alkaloids from the roots of Sophora flavescens and their anti-epatitis B virus activities. J Org Chem. 2016; 81:6273–6280.
Article22. Zhou Y, Wu Y, Deng L, Chen L, Zhao D, Lv L, et al. The alkaloid matrine of the root of Sophora flavescens prevents arrhythmogenic effect of ouabain. Phytomedicine. 2014; 21:931–935.
Article23. Sun N, Sun P, Lv H, Sun Y, Guo J, Wang Z, et al. Matrine displayed antiviral activity in porcine alveolar macrophages co-infected by porcine reproductive and respiratory syndrome virus and porcine circovirus type 2. Sci Rep. 2016; 6:24401.
Article24. Pu J, Fang FF, Li XQ, Shu ZH, Jiang YP, Han T, et al. Matrine exerts a strong anti-arthritic effect on type II collagen-induced arthritis in rats by inhibiting inflammatory responses. Int J Mol Sci. 2016; 17:1410.
Article25. Liu Z, Yang Y, Xu J, Jiang X, Wang J. GW27-e1128 Matrine inhibits cardiac fibrosis by inhibiting TGFβ1/smad signaling pathway. J Am Coll Cardiol. 2016; 68:16 Suppl. C40.26. Mihailova S, Ivanova-Genova E, Lukanov T, Stoyanova V, Milanova V, Naumova E. A study of TNF-α, TGF-β, IL-10, IL-6, and IFN-γ gene polymorphisms in patients with depression. J Neuroimmunol. 2016; 293:123–128.
Article27. Yang P, Wu Z, Huang J, Wang A, Xu S, You P, et al. Regulation of fibroblasts proliferation and function of human hypertrophic scar by Oxymatrine through TGF-β signaling pathway. Chin J Aesth Plast Surg. 2010; 21:557–559.28. Zhang Y, Cui L, Guan G, Wang J, Qiu C, Yang T, et al. Matrine suppresses cardiac fibrosis by inhibiting the TGF-β/Smad pathway in experimental diabetic cardiomyopathy. Mol Med Rep. 2018; 17:1775–1781.
Article29. Zhao N, Pan S, Zhang Y, Liu X, Guan G, Wang J, et al. TGF-β/ Smads signal pathway is involved in the anti-fibrotic effect of matrine and improvement of the cardiac function in the diabetic cardiomyopathy rats. J Shanxi Med Univ. 2015; 13:312–327.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Tripartite Motif Family Proteins in TGF-β Signaling Pathway and Cancer
- TGF-beta-activated kinase-1: New insights into the mechanism of TGF-beta signaling and kidney disease
- Low Level Light Therapy Using an 830-nm Light Emitting Diode Promotes Wound Healing via TGF-β/SMAD Pathway Activation
- Hydrogen sulfide alleviates hypothyroidism-induced myocardial fibrosis in rats through stimulating autophagy and inhibiting TGF-β1/Smad2 pathway
- TGF-β induces Smad2 Phosphorylation, ARE Induction, and Trophoblast Differentiation