Korean J Physiol Pharmacol.
2023 Jan;27(1):1-8. 10.4196/kjpp.2023.27.1.1.
Hydrogen sulfide alleviates hypothyroidism-induced myocardial fibrosis in rats through stimulating autophagy and inhibiting TGF-β1/Smad2 pathway
- Affiliations
-
- 1Department of Cardiology, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan 421001, China
- 2Department of Cardiology, The People's Hospital of Shuangfeng County, Loudi, Hunan 417700, China
- KMID: 2537498
- DOI: http://doi.org/10.4196/kjpp.2023.27.1.1
Abstract
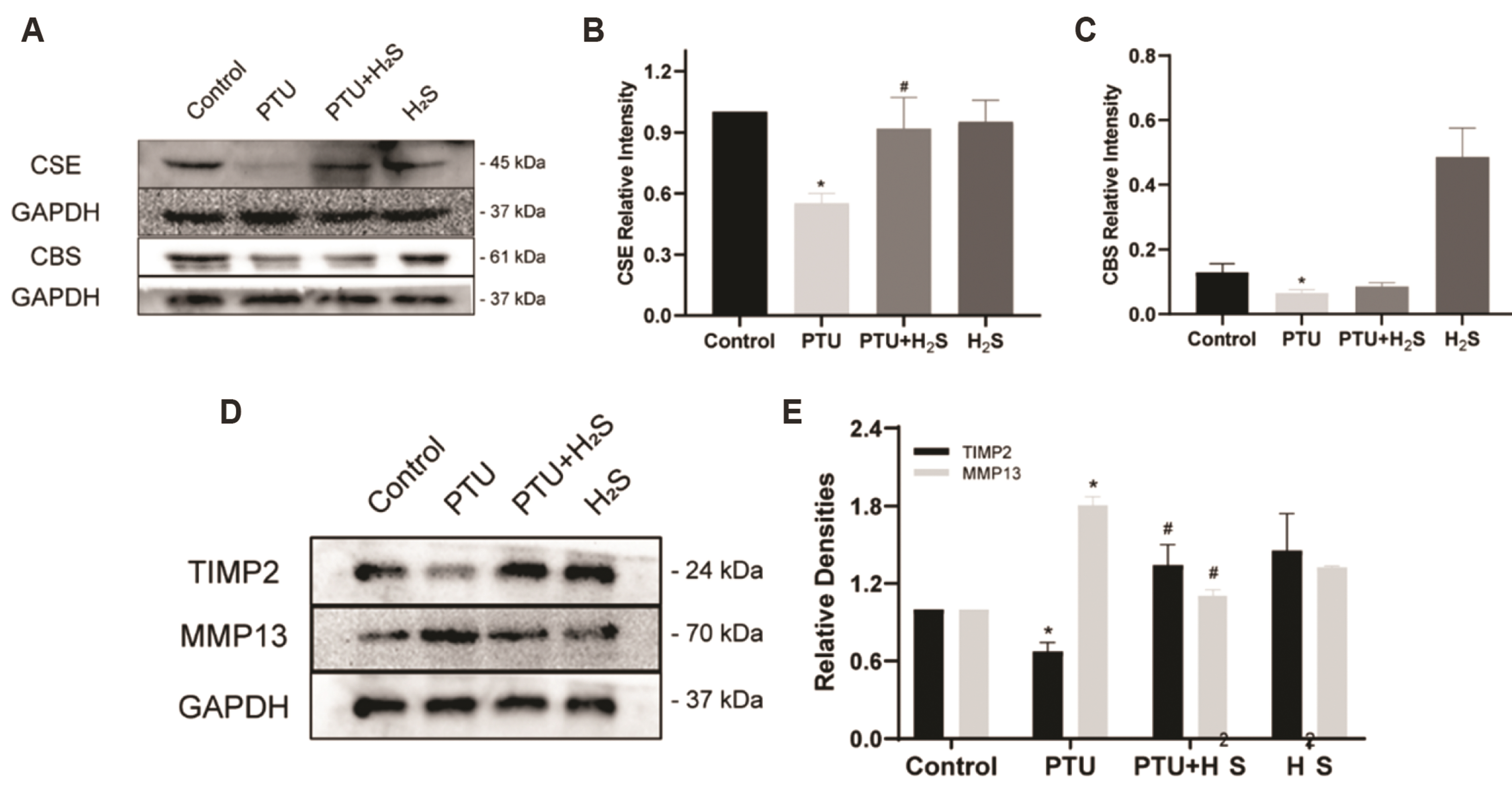
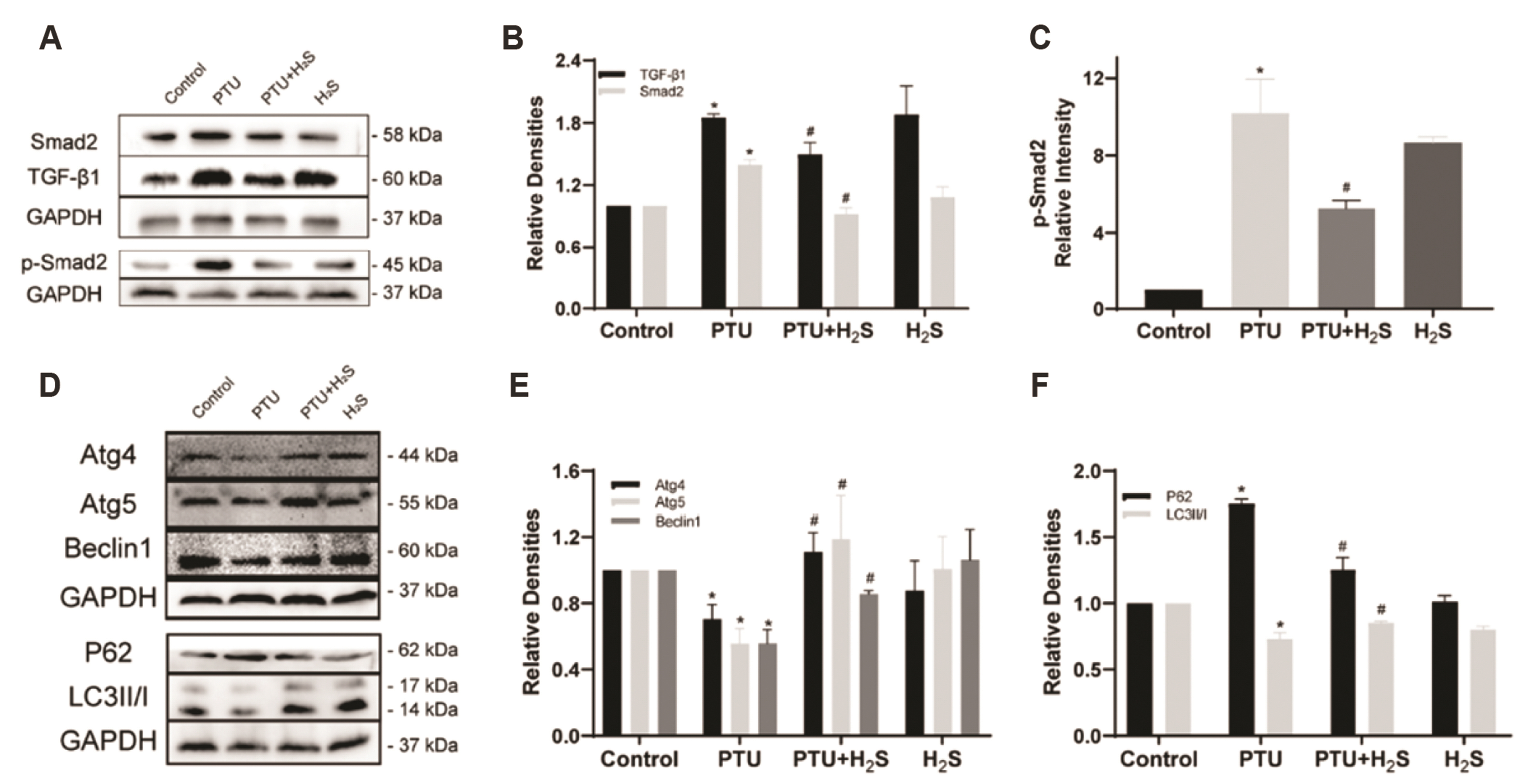
- Hypothyroidism alone can lead to myocardial fibrosis and result in heart failure, but traditional hormone replacement therapy does not improve the fibrotic situation. Hydrogen sulfide (H 2 S), a new gas signaling molecule, possesses antiinflammatory, antioxidant, and anti-fibrotic capabilities. Whether H 2 S could improve hypothyroidism-induced myocardial fibrosis are not yet studied. In our study, H 2 S could decrease collagen deposition in the myocardial tissue of rats caused by hypothyroidism. Furthermore, in hypothyroidism-induced rats, we found that H 2 S could enhance cystathionine-gamma-lyase (CSE), not cystathionine β-synthase (CBS), protein expressions. Finally, we noticed that H 2 S could elevate autophagy levels and inhibit the transforming growth factor-β1 (TGF-β1) signal transduction pathway. In conclusion, our experiments not only suggest that H 2 S could alleviate hypothyroidism-induced myocardial fibrosis by activating autophagy and suppressing TGF-β1/ SMAD family member 2 (Smad 2) signal transduction pathway, but also show that it can be used as a complementary treatment to conventional hormone therapy.
Keyword
Figure
Reference
-
1. Tang YD, Kuzman JA, Said S, Anderson BE, Wang X, Gerdes AM. 2005; Low thyroid function leads to cardiac atrophy with chamber dilatation, impaired myocardial blood flow, loss of arterioles, and severe systolic dysfunction. Circulation. 112:3122–3130. DOI: 10.1161/CIRCULATIONAHA.105.572883. PMID: 16275864. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=30944459664&origin=inward.2. Udovcic M, Pena RH, Patham B, Tabatabai L, Kansara A. 2017; Hypothyroidism and the heart. Methodist Debakey Cardiovasc J. 13:55–59. DOI: 10.14797/mdcj-13-2-55. PMID: 28740582. PMCID: PMC5512679. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85040606695&origin=inward.3. Bano A, Chaker L, Muka T, Mattace-Raso FUS, Bally L, Franco OH, Peeters RP, Razvi S. 2020; Thyroid function and the risk of fibrosis of the liver, heart, and lung in humans: a systematic review and meta-analysis. Thyroid. 30:806–820. DOI: 10.1089/thy.2019.0572. PMID: 31910097. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85083548579&origin=inward.4. Espeland T, Lunde IG, Amundsen BH, Gullestad L, Aakhus S. 2018; Myocardial fibrosis. Tidsskr Nor Laegeforen. doi: 10.4045/tidsskr.17.1027. DOI: 10.4045/tidsskr.17.1027. PMID: 30344312. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85054843772&origin=inward.5. Hajje G, Saliba Y, Itani T, Moubarak M, Aftimos G, Farès N. 2014; Hypothyroidism and its rapid correction alter cardiac remodeling. PLoS One. 9:e109753. DOI: 10.1371/journal.pone.0109753. PMID: 25333636. PMCID: PMC4198123. PMID: 0f1a47ba28374904a46e5bbca4a738ba. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84908102452&origin=inward.6. Liu M, Li Y, Liang B, Li Z, Jiang Z, Chu C, Yang J. 2018; Hydrogen sulfide attenuates myocardial fibrosis in diabetic rats through the JAK/STAT signaling pathway. Int J Mol Med. 41:1867–1876. DOI: 10.3892/ijmm.2018.3419. PMID: 29393353. PMCID: PMC5810211. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85041571148&origin=inward.7. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. 2008; Autophagy fights disease through cellular self-digestion. Nature. 451:1069–1075. DOI: 10.1038/nature06639. PMID: 18305538. PMCID: PMC2670399. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=39849109338&origin=inward.8. Xin W, Yu Y, Ma Y, Gao Y, Xu Y, Chen L, Wan Q. 2017; Thyroid-stimulating hormone stimulation downregulates autophagy and promotes apoptosis in chondrocytes. Endocr J. 64:749–757. DOI: 10.1507/endocrj.EJ16-0534. PMID: 28626114. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85026434988&origin=inward.9. Chen T, Li J, Liu J, Li N, Wang S, Liu H, Zeng M, Zhang Y, Bu P. 2015; Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and improves cardiac function via the TGF-β/Smad3 pathway. Am J Physiol Heart Circ Physiol. 308:H424–H434. DOI: 10.1152/ajpheart.00454.2014. PMID: 25527776. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84928635872&origin=inward.10. Khalil H, Kanisicak O, Prasad V, Correll RN, Fu X, Schips T, Vagnozzi RJ, Liu R, Huynh T, Lee SJ, Karch J, Molkentin JD. 2017; Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest. 127:3770–3783. DOI: 10.1172/JCI94753. PMID: 28891814. PMCID: PMC5617658. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85030553538&origin=inward.11. Xiao T, Luo J, Wu Z, Li F, Zeng O, Yang J. 2016; Effects of hydrogen sulfide on myocardial fibrosis and PI3K/AKT1-regulated autophagy in diabetic rats. Mol Med Rep. 13:1765–1773. DOI: 10.3892/mmr.2015.4689. PMID: 26676365. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84956950631&origin=inward.12. Moon S, Kim MJ, Yu JM, Yoo HJ, Park YJ. 2018; Subclinical hypothyroidism and the risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Thyroid. 28:1101–1110. DOI: 10.1089/thy.2017.0414. PMID: 29978767. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85053431654&origin=inward.13. Baumgartner C, da Costa BR, Collet TH, Feller M, Floriani C, Bauer DC, Cappola AR, Heckbert SR, Ceresini G, Gussekloo J, den Elzen WPJ, Peeters RP, Luben R, Völzke H, Dörr M, Walsh JP, Bremner A, Iacoviello M, Macfarlane P, Heeringa J, et al. 2017; Thyroid function within the normal range, subclinical hypothyroidism, and the risk of atrial fibrillation. Circulation. 136:2100–2116. DOI: 10.1161/CIRCULATIONAHA.117.028753. PMID: 29061566. PMCID: PMC5705446. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85037357547&origin=inward.14. Li Y, Liu M, Yi J, Song X, Zheng X, Liu D, Wang S, Chu C, Yang J. 2020; Exogenous hydrogen sulfide inhibits apoptosis by regulating endoplasmic reticulum stress-autophagy axis and improves myocardial reconstruction after acute myocardial infarction. Acta Biochim Biophys Sin (Shanghai). 52:1325–1336. DOI: 10.1093/abbs/gmaa133. PMID: 33210714. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85099171393&origin=inward.15. Daliang Z, Lifang Y, Hong F, Lingling Z, Lin W, Dapeng L, Tianshu Z, Weimin L. 2019; Netrin-1 plays a role in the effect of moderate exercise on myocardial fibrosis in rats. PLoS One. 14:e0199802. DOI: 10.1371/journal.pone.0199802. PMID: 30789913. PMCID: PMC6383912. PMID: c8109acc83a04a2cae90efcee507672b. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85061921057&origin=inward.16. Sun Y, Yao X, Zhang QJ, Zhu M, Liu ZP, Ci B, Xie Y, Carlson D, Rothermel BA, Sun Y, Levine B, Hill JA, Wolf SE, Minei JP, Zang QS. 2018; Beclin-1-dependent autophagy protects the heart during sepsis. Circulation. 138:2247–2262. DOI: 10.1161/CIRCULATIONAHA.117.032821. PMID: 29853517. PMCID: PMC6274625. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85056463995&origin=inward.17. Éva Sikura K, Combi Z, Potor L, Szerafin T, Hendrik Z, Méhes G, Gergely P, Whiteman M, Beke L, Fürtös I, Balla G, Balla J. 2020; Hydrogen sulfide inhibits aortic valve calcification in heart via regulating RUNX2 by NF-κB, a link between inflammation and mineralization. J Adv Res. 27:165–176. DOI: 10.1016/j.jare.2020.07.005. PMID: 33318875. PMCID: PMC7728582. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85089007325&origin=inward.18. Ellmers LJ, Templeton EM, Pilbrow AP, Frampton C, Ishii I, Moore PK, Bhatia M, Richards AM, Cameron VA. 2020; Hydrogen sulfide treatment improves post-infarct remodeling and long-term cardiac function in CSE knockout and wild-type mice. Int J Mol Sci. 21:4284. DOI: 10.3390/ijms21124284. PMID: 32560137. PMCID: PMC7352717. PMID: c51f5e3d17634967ac531beea091721c. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85086753456&origin=inward.19. Pan LL, Liu XH, Shen YQ, Wang NZ, Xu J, Wu D, Xiong QH, Deng HY, Huang GY, Zhu YZ. 2013; Inhibition of NADPH oxidase 4-related signaling by sodium hydrosulfide attenuates myocardial fibrotic response. Int J Cardiol. 168:3770–3778. DOI: 10.1016/j.ijcard.2013.06.007. PMID: 23830348. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84886234752&origin=inward.20. Yu XH, Cui LB, Wu K, Zheng XL, Cayabyab FS, Chen ZW, Tang CK. 2014; Hydrogen sulfide as a potent cardiovascular protective agent. Clin Chim Acta. 437:78–87. DOI: 10.1016/j.cca.2014.07.012. PMID: 25058799. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84905279994&origin=inward.21. Sciarretta S, Yee D, Nagarajan N, Bianchi F, Saito T, Valenti V, Tong M, Del Re DP, Vecchione C, Schirone L, Forte M, Rubattu S, Shirakabe A, Boppana VS, Volpe M, Frati G, Zhai P, Sadoshima J. 2018; Trehalose-induced activation of autophagy improves cardiac remodeling after myocardial infarction. J Am Coll Cardiol. 71:1999–2010. DOI: 10.1016/j.jacc.2018.02.066. PMID: 29724354. PMCID: PMC6347412. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85046540313&origin=inward.22. Dewanjee S, Vallamkondu J, Kalra RS, John A, Reddy PH, Kandimalla R. 2021; Autophagy in the diabetic heart: a potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res Rev. 68:101338. DOI: 10.1016/j.arr.2021.101338. PMID: 33838320. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85104070130&origin=inward.23. Zhang QY, Jin HF, Chen S, Chen QH, Tang CS, Du JB, Huang YQ. 2018; Hydrogen sulfide regulating myocardial structure and function by targeting cardiomyocyte autophagy. Chin Med J (Engl). 131:839–844. DOI: 10.4103/0366-6999.228249. PMID: 29578128. PMCID: PMC5887743. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85044469762&origin=inward.24. Lv S, Yuan P, Dong J, Lu C, Li M, Qu F, Zhu Y, Yuan Z, Zhang J. 2020; QiShenYiQi pill improves the reparative myocardial fibrosis by regulating autophagy. J Cell Mol Med. 24:11283–11293. DOI: 10.1111/jcmm.15695. PMID: 32881330. PMCID: PMC7576289. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85090164068&origin=inward.25. Luo LF, Qin LY, Wang JX, Guan P, Wang N, Ji ES. 2021; Astragaloside IV attenuates the myocardial injury caused by adriamycin by inhibiting autophagy. Front Pharmacol. 12:669782. DOI: 10.3389/fphar.2021.669782. PMID: 34108879. PMCID: PMC8184095. PMID: e008cd6af601493f8ef7b01b0465125d. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85107408654&origin=inward.26. Margariti A, Li H, Chen T, Martin D, Vizcay-Barrena G, Alam S, Karamariti E, Xiao Q, Zampetaki A, Zhang Z, Wang W, Jiang Z, Gao C, Ma B, Chen YG, Cockerill G, Hu Y, Xu Q, Zeng L. 2013; XBP1 mRNA splicing triggers an autophagic response in endothelial cells through BECLIN-1 transcriptional activation. J Biol Chem. 288:859–872. DOI: 10.1074/jbc.M112.412783. PMID: 23184933. PMCID: PMC3543035. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84872311621&origin=inward.27. Maruyama T, Noda NN. 2017; Autophagy-regulating protease Atg4: structure, function, regulation and inhibition. J Antibiot (Tokyo). 71:72–78. DOI: 10.1038/ja.2017.104. PMID: 28901328. PMCID: PMC5799747. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85040256286&origin=inward.28. Luo S, Rubinsztein DC. 2007; Atg5 and Bcl-2 provide novel insights into the interplay between apoptosis and autophagy. Cell Death Differ. 14:1247–1250. DOI: 10.1038/sj.cdd.4402149. PMID: 17431417. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34250705747&origin=inward.29. Danieli A, Martens S. 2018; p62-mediated phase separation at the intersection of the ubiquitin-proteasome system and autophagy. J Cell Sci. 131:jcs214304. DOI: 10.1242/jcs.214304. PMID: 30287680. PMCID: PMC7610772. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85054449738&origin=inward.30. Tanida I, Ueno T, Kominami E. 2008; LC3 and autophagy. Methods Mol Biol. 445:77–88. DOI: 10.1007/978-1-59745-157-4_4. PMID: 18425443. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84934440388&origin=inward.31. Mizushima N, Yoshimori T. 2007; How to interpret LC3 immunoblotting. Autophagy. 3:542–545. DOI: 10.4161/auto.4600. PMID: 17611390. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=35848967804&origin=inward.32. Li Y, Liu R, Wu J, Li X. 2020; Self-eating: friend or foe? The emerging role of autophagy in fibrotic diseases. Theranostics. 10:7993–8017. DOI: 10.7150/thno.47826. PMID: 32724454. PMCID: PMC7381749. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85088882351&origin=inward.33. Guo Y, Gupte M, Umbarkar P, Singh AP, Sui JY, Force T, Lal H. 2017; Entanglement of GSK-3β, β-catenin and TGF-β1 signaling network to regulate myocardial fibrosis. J Mol Cell Cardiol. 110:109–120. DOI: 10.1016/j.yjmcc.2017.07.011. PMID: 28756206. PMCID: PMC5581678. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85026542006&origin=inward.34. Su SA, Yang D, Wu Y, Xie Y, Zhu W, Cai Z, Shen J, Fu Z, Wang Y, Jia L, Wang Y, Wang JA, Xiang M. 2017; EphrinB2 regulates cardiac fibrosis through modulating the interaction of Stat3 and TGF-β/Smad3 signaling. Circ Res. 121:617–627. DOI: 10.1161/CIRCRESAHA.117.311045. PMID: 28743805. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85026449025&origin=inward.35. Gao H, Bo Z, Wang Q, Luo L, Zhu H, Ren Y. 2019; Salvanic acid B inhibits myocardial fibrosis through regulating TGF-β1/Smad signaling pathway. Biomed Pharmacother. 110:685–691. DOI: 10.1016/j.biopha.2018.11.098. PMID: 30553195. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85058166395&origin=inward.36. Shi YX, Chen Y, Zhu YZ, Huang GY, Moore PK, Huang SH, Yao T, Zhu YC. 2007; Chronic sodium hydrosulfide treatment decreases medial thickening of intramyocardial coronary arterioles, interstitial fibrosis, and ROS production in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 293:H2093–H2100. DOI: 10.1152/ajpheart.00088.2007. PMID: 17630351. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=35348993710&origin=inward.37. Chan EC, Peshavariya HM, Liu GS, Jiang F, Lim SY, Dusting GJ. 2013; Nox4 modulates collagen production stimulated by transforming growth factor β1 in vivo and in vitro. Biochem Biophys Res Commun. 430:918–925. DOI: 10.1016/j.bbrc.2012.11.138. PMID: 23261430. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84872491620&origin=inward.38. Xue H, Yuan P, Ni J, Li C, Shao D, Liu J, Shen Y, Wang Z, Zhou L, Zhang W, Huang Y, Yu C, Wang R, Lu L. 2013; H2S inhibits hyperglycemia-induced intrarenal renin-angiotensin system activation via attenuation of reactive oxygen species generation. PLoS One. 8:e74366. DOI: 10.1371/journal.pone.0074366. PMID: 24058553. PMCID: PMC3772925. PMID: 6fe2d37d566c4eecbdc1307bdd192d1b. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84884160965&origin=inward.39. Kumar M, Arora P, Sandhir R. 2021; Hydrogen sulfide reverses LPS-induced behavioral deficits by suppressing microglial activation and promoting M2 polarization. J Neuroimmune Pharmacol. 16:483–499. DOI: 10.1007/s11481-020-09920-z. PMID: 32676889. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85088039180&origin=inward.40. Yuan P, Xue H, Zhou L, Qu L, Li C, Wang Z, Ni J, Yu C, Yao T, Huang Y, Wang R, Lu L. 2011; Rescue of mesangial cells from high glucose-induced over-proliferation and extracellular matrix secretion by hydrogen sulfide. Nephrol Dial Transplant. 26:2119–2126. DOI: 10.1093/ndt/gfq749. PMID: 21208996. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79960002418&origin=inward.41. Lu M, Qin Q, Yao J, Sun L, Qin X. 2019; Induction of LOX by TGF-β1/Smad/AP-1 signaling aggravates rat myocardial fibrosis and heart failure. IUBMB Life. 71:1729–1739. DOI: 10.1002/iub.2112. PMID: 31317653. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85069892187&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hydrogen sulfide ameliorates abdominal aorta coarctationinduced myocardial fibrosis by inhibiting pyroptosis through regulating eukaryotic translation initiation factor 2αα phosphorylation and activating PI3K/AKT1 pathway
- The flavonoid fisetin ameliorates renal fibrosis by inhibiting SMAD3 phosphorylation, oxidative damage, and inflammation in ureteral obstructed kidney in mice
- Effect of FTY-720 on Pulmonary Fibrosis in Mice via the TGF-β1 Signaling Pathway and Autophagy
- Mangiferin ameliorates cardiac fibrosis in D-galactose-induced aging rats by inhibiting TGF-β/p38/MK2 signaling pathway
- Extracellular Vesicles Derived from Adipose Stem Cells Alleviate Systemic Sclerosis by Inhibiting TGF-β Pathway