Int J Stem Cells.
2018 Jun;11(1):111-120. 10.15283/ijsc17069.
TGF-β induces Smad2 Phosphorylation, ARE Induction, and Trophoblast Differentiation
- Affiliations
-
- 1Department of Neuroscience, Cell Biology and Physiology, Wright State University Boonshoft School of Medicine, Dayton, Ohio 45435, USA. Thomas.L.brown@wright.edu
- 2Department of Reproductive Medicine, University of California-San Diego, San Diego, California 92093, USA.
- KMID: 2413507
- DOI: http://doi.org/10.15283/ijsc17069
Abstract
- BACKGROUND
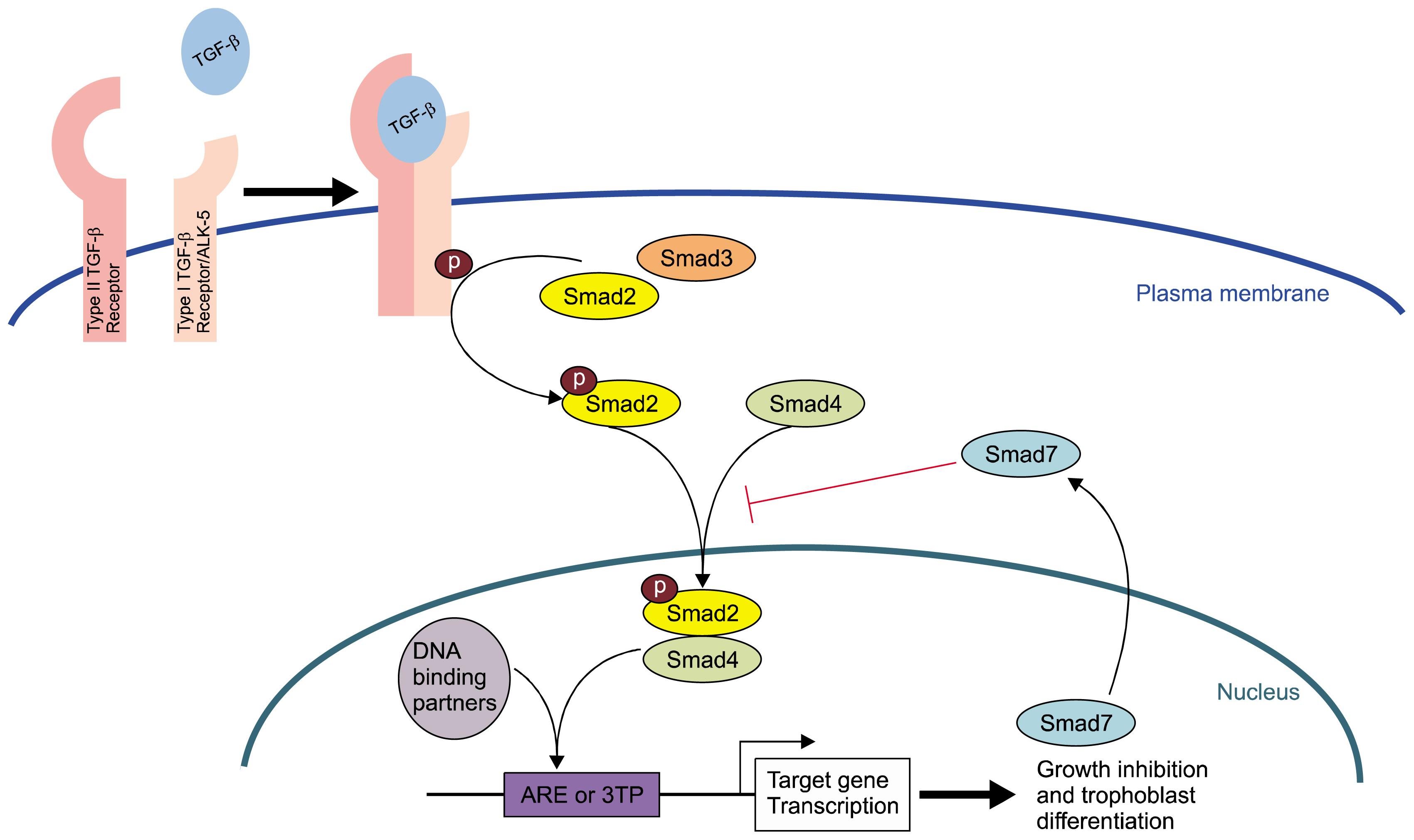
Transforming growth factor beta (TGF-β) signaling has been shown to control a large number of critical cellular actions such as cell death, differentiation, and development and has been implicated as a major regulator of placental function. SM10 cells are a mouse placental progenitor cell line, which has been previously shown to differentiate into nutrient transporting, labyrinthine-like cells upon treatment with TGF-β. However, the signal transduction pathway activated by TGF-β to induce SM10 progenitor differentiation has yet to be fully investigated.
MATERIALS AND METHODS
In this study the SM10 labyrinthine progenitor cell line was used to investigate TGF-β induced differentiation. Activation of the TGF-β pathway and the ability of TGF-β to induce differentiation were investigated by light microscopy, luciferase assays, and Western blot analysis.
RESULTS
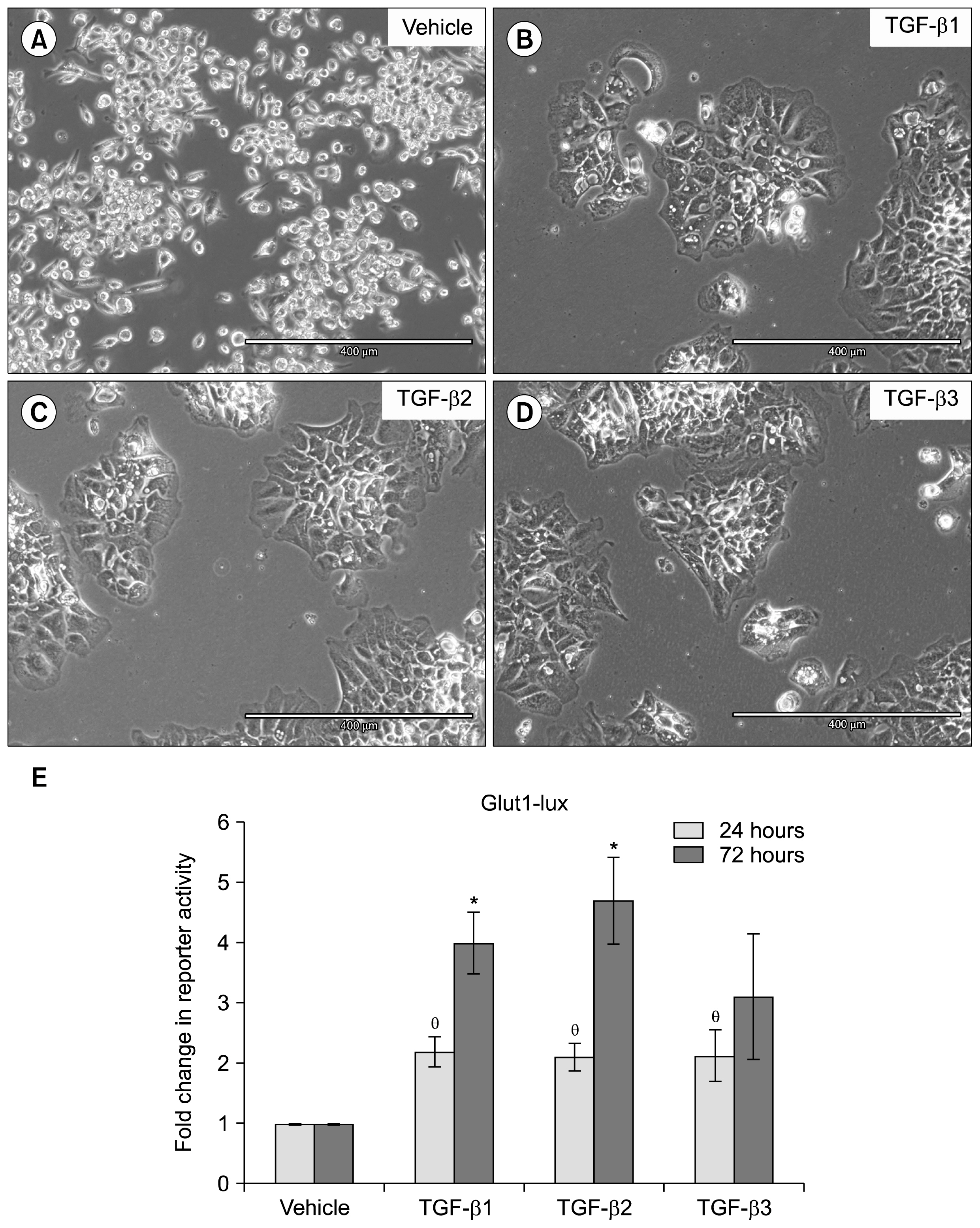
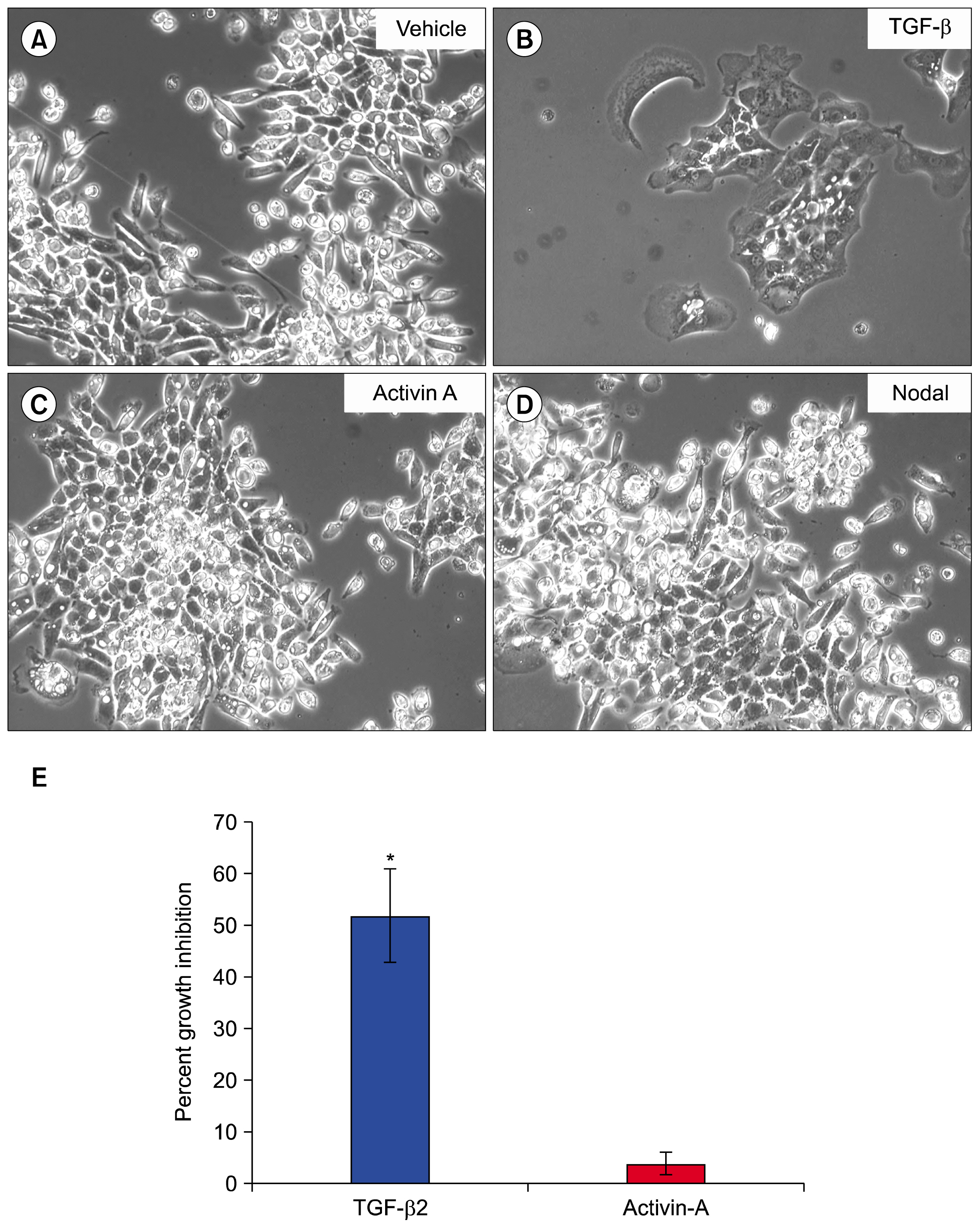
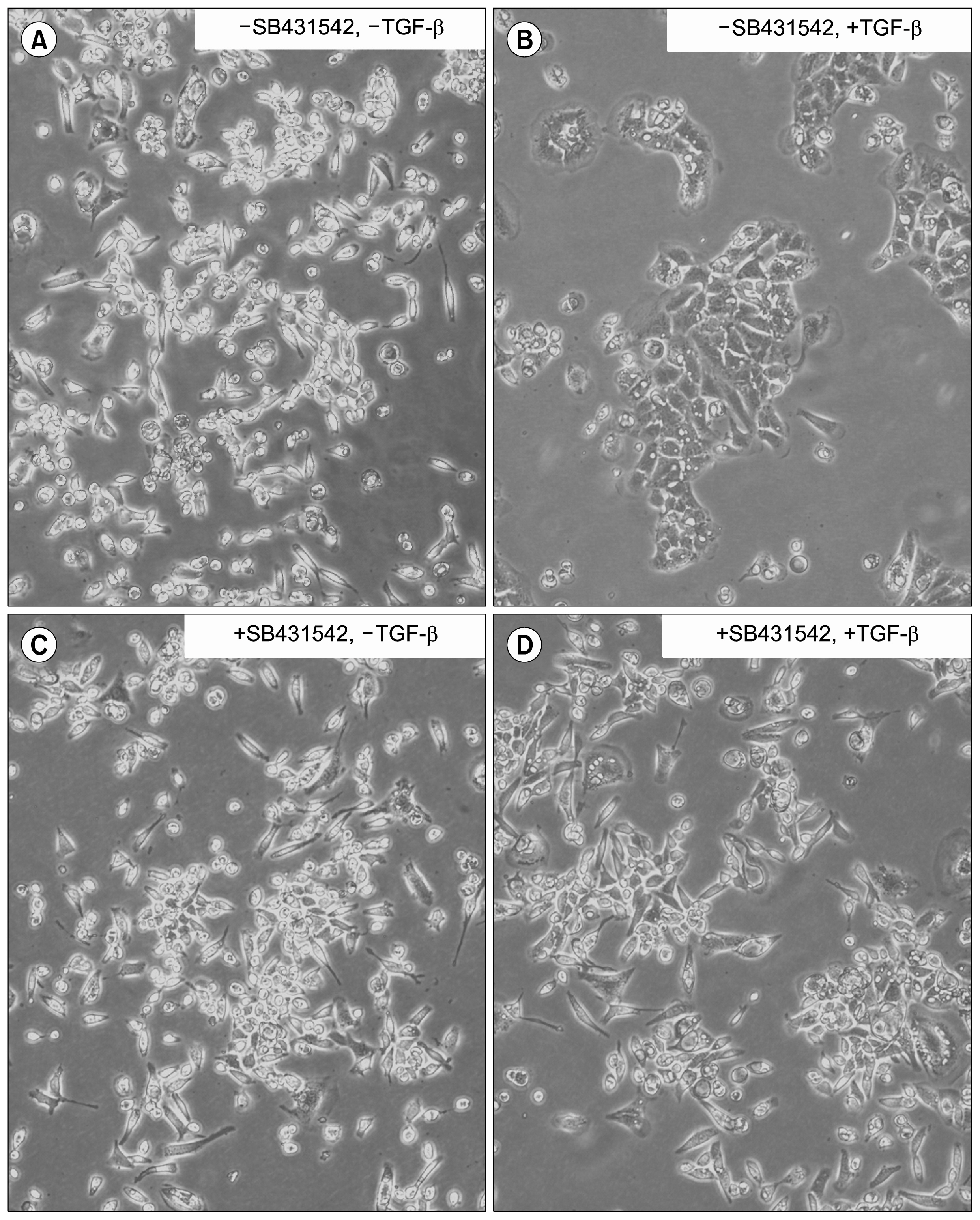
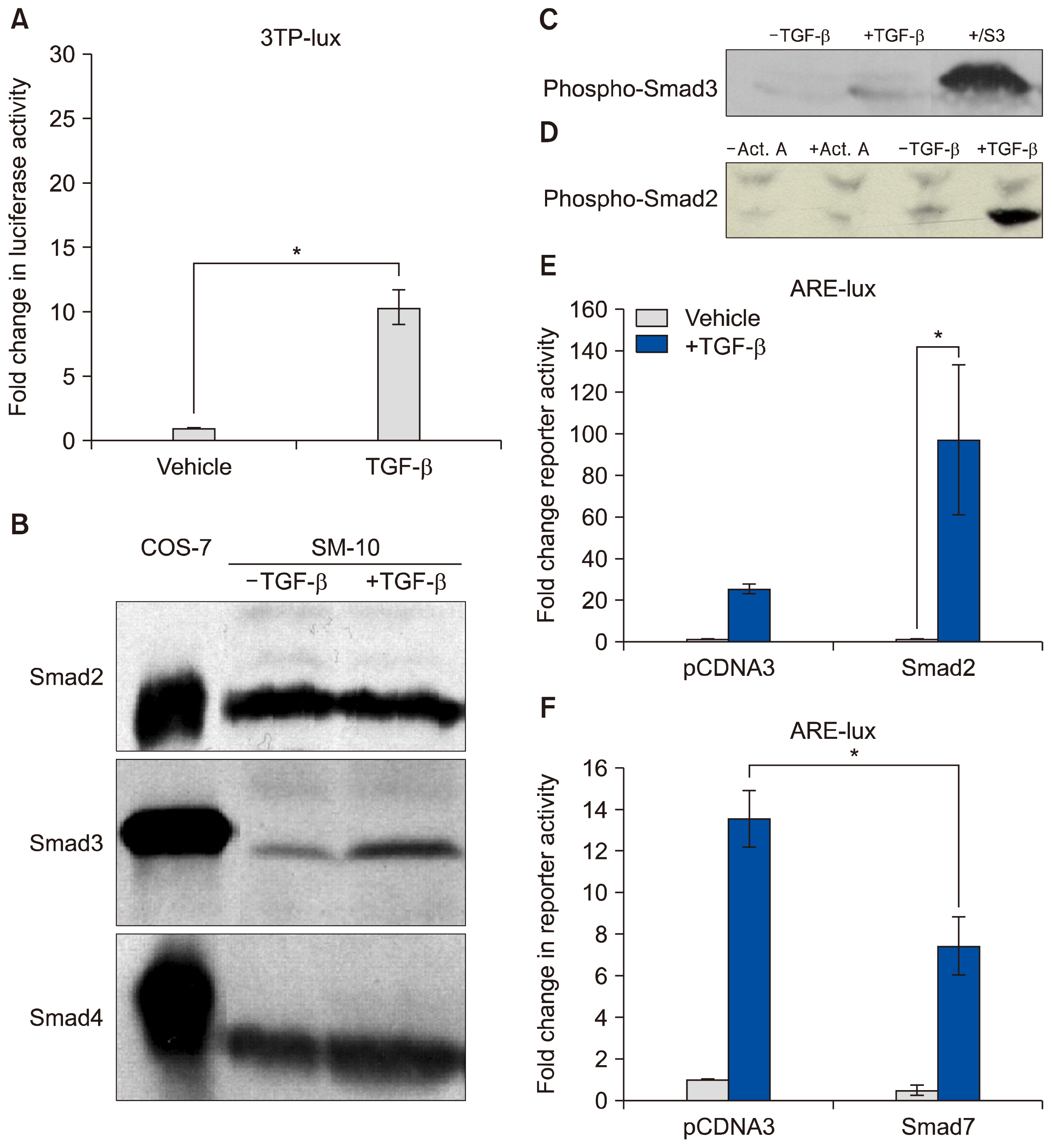
AND CONCLUSIONS: In this report, we show that three isoforms of TGF-β have the ability to terminally differentiate SM10 cells, whereas other predominant members of the TGF-β superfamily, Nodal and Activin A, do not. Additionally, we have determined that TGF-β induced Smad2 phosphorylation can be mediated via the ALK-5 receptor with subsequent transactivation of the Activin response element. Our studies identify an important regulatory signaling pathway in SM10 progenitor cells that is involved in labyrinthine trophoblast differentiation.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Vaughan OR, Rosario FJ, Powell TL, Jansson T. Regulation of placental amino acid transport and fetal growth. Prog Mol Biol Transl Sci. 2017; 145:217–251. DOI: 10.1016/bs.pmbts.2016.12.008. PMID: 28110752.
Article2. Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000; 14:3191–3203. DOI: 10.1101/gad.853700. PMID: 11124810. PMCID: 317149.
Article3. Ganguly A, Touma M, Thamotharan S, De Vivo DC, Devaskar SU. Maternal calorie restriction causing uteroplacental insufficiency differentially affects mammalian placental glucose and leucine transport molecular mechanisms. Endocrinology. 2016; 157:4041–4054. DOI: 10.1210/en.2016-1259. PMID: 27494059. PMCID: 5045505.
Article4. Shaarawy M, El Meleigy M, Rasheed K. Maternal serum transforming growth factor beta-2 in preeclampsia and eclampsia, a potential biomarker for the assessment of disease severity and fetal outcome. J Soc Gynecol Investig. 2001; 8:27–31. DOI: 10.1177/107155760100800105. PMID: 11223354.
Article5. Xu J, Sivasubramaniyam T, Yinon Y, Tagliaferro A, Ray J, Nevo O, Post M, Caniggia I. Aberrant TGFβ signaling contributes to altered trophoblast differentiation in preeclampsia. Endocrinology. 2016; 157:883–899. DOI: 10.1210/en.2015-1696.
Article6. Gormley M, Ona K, Kapidzic M, Garrido-Gomez T, Zdravkovic T, Fisher SJ. Preeclampsia: novel insights from global RNA profiling of trophoblast subpopulations. Am J Obstet Gynecol. 2017; 217:200.e1–200.e17. DOI: 10.1016/j.ajog.2017.03.017.
Article7. Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012; 13:616–630. DOI: 10.1038/nrm3434.
Article8. Brown TL, Patil S, Howe PH. Analysis of TGF-beta-inducible apoptosis. Methods Mol Biol. 2000; 142:149–167. PMID: 10806621.9. Larsson J, Goumans MJ, Sjöstrand LJ, van Rooijen MA, Ward D, Levéen P, Xu X, ten Dijke P, Mummery CL, Karlsson S. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J. 2001; 20:1663–1673. DOI: 10.1093/emboj/20.7.1663. PMID: 11285230. PMCID: 145465.
Article10. Oshima M, Oshima H, Taketo MM. TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol. 1996; 179:297–302. DOI: 10.1006/dbio.1996.0259. PMID: 8873772.
Article11. Budi EH, Duan D, Derynck R. Transforming growth Factor-β receptors and smads: regulatory complexity and functional versatility. Trends Cell Biol. 2017; 27:658–672. DOI: 10.1016/j.tcb.2017.04.005. PMID: 28552280.
Article12. Goumans MJ, Mummery C. Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol. 2000; 44:253–265. PMID: 10853822.13. Tojo M, Takebe A, Takahashi S, Tanaka K, Imamura T, Miyazono K, Chiba T. Smad7-deficient mice show growth retardation with reduced viability. J Biochem. 2012; 151:621–631. DOI: 10.1093/jb/mvs022. PMID: 22383537.
Article14. Patil S, Wildey GM, Brown TL, Choy L, Derynck R, Howe PH. Smad7 is induced by CD40 and protects WEHI 231 B-lymphocytes from transforming growth factor-beta -induced growth inhibition and apoptosis. J Biol Chem. 2000; 275:38363–38370. DOI: 10.1074/jbc.M004861200. PMID: 10995749.
Article15. Soncin F, Natale D, Parast MM. Signaling pathways in mouse and human trophoblast differentiation: a comparative review. Cell Mol Life Sci. 2015; 72:1291–1302. DOI: 10.1007/s00018-014-1794-x. PMCID: 4366325.
Article16. Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology (Bethesda). 2005; 20:180–193.
Article17. Simmons DG, Natale DR, Begay V, Hughes M, Leutz A, Cross JC. Early patterning of the chorion leads to the trilaminar trophoblast cell structure in the placental labyrinth. Development. 2008; 135:2083–2091. DOI: 10.1242/dev.020099. PMID: 18448564. PMCID: 3159581.
Article18. Sharma RK. Mouse trophoblastic cell lines: I--Relationship between invasive potential and TGF-beta 1. In Vivo. 1998; 12:431–440. PMID: 9827348.19. Selesniemi K, Reedy M, Gultice A, Guilbert LJ, Brown TL. Transforming growth factor-beta induces differentiation of the labyrinthine trophoblast stem cell line SM10. Stem Cells Dev. 2005; 14:697–711. DOI: 10.1089/scd.2005.14.697.
Article20. Selesniemi K, Albers RE, Brown TL. Id2 Mediates differentiation of labyrinthine placental progenitor cell line, SM10. Stem Cells Dev. 2016; 25:959–974. DOI: 10.1089/scd.2016.0010. PMID: 27168216. PMCID: 4931356.
Article21. Waker CA, Albers RE, Pye RL, Doliboa SR, Wyatt CN, Brown TL, Mayes DA. AMPK knockdown in placental labyrinthine progenitor cells results in restriction of critical energy resources and terminal differentiation failure. Stem Cells Dev. 2017; 26:808–817. DOI: 10.1089/scd.2016.0252. PMID: 28335680. PMCID: 5466016.
Article22. Hocevar BA, Brown TL, Howe PH. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999; 18:1345–1356. DOI: 10.1093/emboj/18.5.1345. PMID: 10064600. PMCID: 1171224.
Article23. Cárcamo J, Weis FM, Ventura F, Wieser R, Wrana JL, Attisano L, Massagué J. Type I receptors specify growth-inhibitory and transcriptional responses to transforming growth factor beta and activin. Mol Cell Biol. 1994; 14:3810–3821. DOI: 10.1128/MCB.14.6.3810. PMID: 8196624. PMCID: 358748.
Article24. Wang G, Matsuura I, He D, Liu F. Transforming growth factor-{beta}-inducible phosphorylation of Smad3. J Biol Chem. 2009; 284:9663–9673. DOI: 10.1074/jbc.M809281200. PMID: 19218245. PMCID: 2665087.25. Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis. 2003; 8:345–352. DOI: 10.1023/A:1024116916932. PMID: 12815277.26. Selesniemi K, Brown TL. Metafectene Technical Note-SM10 transfection [Internet]. 2004. Available from: http://www.biontex.com/media/references/SM10_transfection_plasmid_DNA_Metafectene_Biontex_Browne2.pdf.27. Doran DM, Kulkarni-Datar K, Cool DR, Brown TL. Hypoxia activates constitutive luciferase reporter constructs. Biochimie. 2011; 93:361–368. DOI: 10.1016/j.biochi.2010.10.009. PMCID: 3020074.
Article28. Kitagawa T, Masumi A, Akamatsu Y. Transforming growth factor-beta 1 stimulates glucose uptake and the expression of glucose transporter mRNA in quiescent Swiss mouse 3T3 cells. J Biol Chem. 1991; 266:18066–18071. PMID: 1917944.
Article29. Inoki K, Haneda M, Maeda S, Koya D, Kikkawa R. TGF-beta 1 stimulates glucose uptake by enhancing GLUT1 expression in mesangial cells. Kidney Int. 1999; 55:1704–1712. DOI: 10.1046/j.1523-1755.1999.00438.x. PMID: 10231432.
Article30. Inman GJ, Nicolás FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002; 62:65–74. DOI: 10.1124/mol.62.1.65. PMID: 12065756.
Article31. Weiss A, Attisano L. The TGFbeta superfamily signaling pathway. Wiley Interdiscip Rev Dev Biol. 2013; 2:47–63. DOI: 10.1002/wdev.86. PMID: 23799630.
Article32. Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007; 8:970–982. DOI: 10.1038/nrm2297. PMID: 18000526.
Article33. Moses HL, Roberts AB, Derynck R. The discovery and early days of TGF-β: a historical perspective. Cold Spring Harb Perspect Biol. 2016; 8:a021865. DOI: 10.1101/cshperspect.a021865.
Article34. Wilkes MC, Murphy SJ, Garamszegi N, Leof EB. Cell-type-specific activation of PAK2 by transforming growth factor beta independent of Smad2 and Smad3. Mol Cell Biol. 2003; 23:8878–8889. DOI: 10.1128/MCB.23.23.8878-8889.2003. PMID: 14612425. PMCID: 262664.
Article35. Zhou S, Zawel L, Lengauer C, Kinzler KW, Vogelstein B. Characterization of human FAST-1, a TGF beta and activin signal transducer. Mol Cell. 1998; 2:121–127. DOI: 10.1016/S1097-2765(00)80120-3. PMID: 9702198.36. Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, Kern SE. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell. 1998; 1:611–617. DOI: 10.1016/S1097-2765(00)80061-1. PMID: 9660945.
Article37. Yan X, Liao H, Cheng M, Shi X, Lin X, Feng XH, Chen YG. Smad7 protein interacts with receptor-regulated smads (R-Smads) to inhibit transforming growth factor-β (TGF-β)/smad signaling. J Biol Chem. 2016; 291:382–392. DOI: 10.1074/jbc.M115.694281.
Article38. Natale DR, Hemberger M, Hughes M, Cross JC. Activin promotes differentiation of cultured mouse trophoblast stem cells towards a labyrinth cell fate. Dev Biol. 2009; 335:120–131. DOI: 10.1016/j.ydbio.2009.08.022. PMID: 19716815.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Retinoic Acid and MAPK Inhibitors on Phosphorylation of Smad2/3 Induced by Transforming Growth Factor β1
- 4-O-Methylhonokiol Protects HaCaT Cells from TGF-β1-Induced Cell Cycle Arrest by Regulating Canonical and Non-Canonical Pathways of TGF-β Signaling
- Simvastatin inhibits sphingosylphosphorylcholine-induced differentiation of human mesenchymal stem cells into smooth muscle cells
- Expression of Epidermal Growth Factor, Transforming Growth Factor-alphaand Epidermal Growth Factor Receptor in Human Trophoblast and Decidua
- TGF-β Signalling is Suppressed under Pro-Hypertrophic Conditions in MSC Chondrogenesis Due to TGF-β Receptor Downregulation