Yonsei Med J.
2016 Nov;57(6):1461-1467. 10.3349/ymj.2016.57.6.1461.
Suppression of MicroRNA let-7a Expression by Agmatine Regulates Neural Stem Cell Differentiation
- Affiliations
-
- 1Department of Anatomy, Yonsei University College of Medicine, Seoul, Korea. jelee@yuhs.ac
- 2Brain Korea 21 Plus Project for Medical Sciences and Brain Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2427165
- DOI: http://doi.org/10.3349/ymj.2016.57.6.1461
Abstract
- PURPOSE
Neural stem cells (NSCs) effectively reverse some severe central nervous system (CNS) disorders, due to their ability to differentiate into neurons. Agmatine, a biogenic amine, has cellular protective effects and contributes to cellular proliferation and differentiation in the CNS. Recent studies have elucidated the function of microRNA let-7a (let-7a) as a regulator of cell differentiation with roles in regulating genes associated with CNS neurogenesis.
MATERIALS AND METHODS
This study aimed to investigate whether agmatine modulates the expression of crucial regulators of NSC differentiation including DCX, TLX, c-Myc, and ERK by controlling let-7a expression.
RESULTS
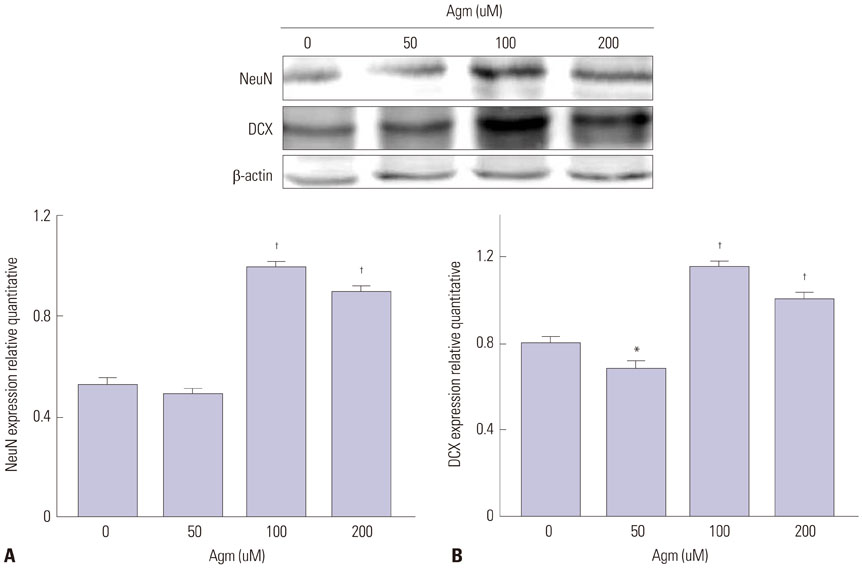
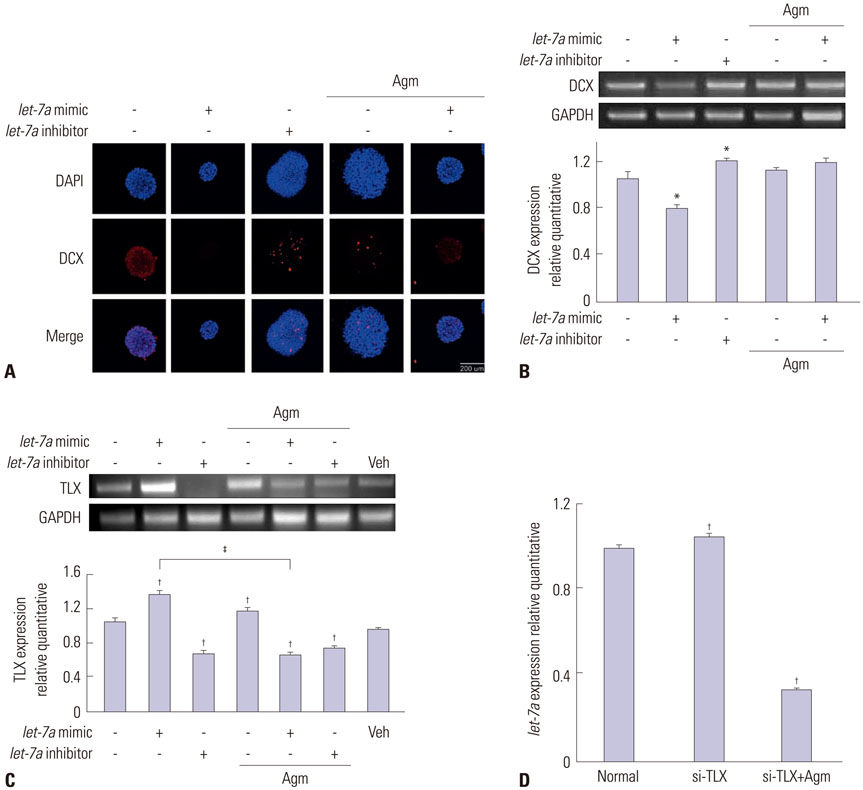
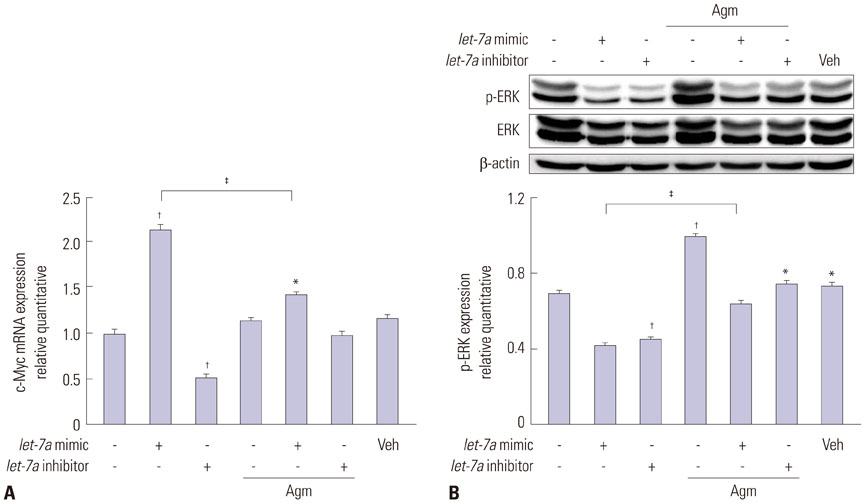
Our data suggest that high levels of let-7a promoted the expression of TLX and c-Myc, as well as repressed DCX and ERK expression. In addition, agmatine attenuated expression of TLX and increased expression of ERK by negatively regulating let-7a.
CONCLUSION
Our study therefore enhances the present understanding of the therapeutic potential of NSCs in CNS disorders.
Keyword
MeSH Terms
Figure
Reference
-
1. Yao J, Mu Y, Gage FH. Neural stem cells: mechanisms and modeling. Protein Cell. 2012; 3:251–261.
Article2. Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011; 70:687–702.
Article3. Kazanis I, Lathia J, Moss L, ffrench-Constant C. The neural stem cell microenvironment. Cambridge: StemBook;2008.4. Joo KM, Kang BG, Yeon JY, Cho YJ, An JY, Song HS, et al. Experimental and clinical factors influencing long-term stable in vitro expansion of multipotent neural cells from human adult temporal lobes. Exp Neurol. 2013; 240:168–177.
Article5. Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003; 13:543–550.
Article6. Kallur T, Darsalia V, Lindvall O, Kokaia Z. Human fetal cortical and striatal neural stem cells generate region-specific neurons in vitro and differentiate extensively to neurons after intrastriatal transplantation in neonatal rats. J Neurosci Res. 2006; 84:1630–1644.
Article7. Scadden DT. The stem-cell niche as an entity of action. Nature. 2006; 441:1075–1079.
Article8. Bokara KK, Kwon KH, Nho Y, Lee WT, Park KA, Lee JE. Retroviral expression of arginine decarboxylase attenuates oxidative burden in mouse cortical neural stem cells. Stem Cells Dev. 2011; 20:527–537.
Article9. Reis DJ, Regunathan S. Is agmatine a novel neurotransmitter in brain? Trends Pharmacol Sci. 2000; 21:187–193.
Article10. Kim JH, Yenari MA, Giffard RG, Cho SW, Park KA, Lee JE. Agmatine reduces infarct area in a mouse model of transient focal cerebral ischemia and protects cultured neurons from ischemia-like injury. Exp Neurol. 2004; 189:122–130.
Article11. Mun CH, Lee WT, Park KA, Lee JE. Regulation of endothelial nitric oxide synthase by agmatine after transient global cerebral ischemia in rat brain. Anat Cell Biol. 2010; 43:230–240.
Article12. Song HW, Kumar BK, Kim SH, Jeon YH, Lee YA, Lee WT, et al. Agmatine enhances neurogenesis by increasing ERK1/2 expression, and suppresses astrogenesis by decreasing BMP 2,4 and SMAD 1,5,8 expression in subventricular zone neural stem cells. Life Sci. 2011; 89:439–449.
Article13. Navarro A, Monzo M. MicroRNAs in human embryonic and cancer stem cells. Yonsei Med J. 2010; 51:622–632.
Article14. Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009; 12:399–408.
Article15. Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008; 18:505–516.
Article16. Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000; 408:86–89.
Article17. Zhao C, Sun G, Li S, Lang MF, Yang S, Li W, et al. MicroRNA let-7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proc Natl Acad Sci U S A. 2010; 107:1876–1881.
Article18. Cimadamore F, Amador-Arjona A, Chen C, Huang CT, Terskikh AV. SOX2-LIN28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proc Natl Acad Sci U S A. 2013; 110:E3017–E3026.
Article19. Zhao C, Sun G, Ye P, Li S, Shi Y. MicroRNA let-7d regulates the TLX/microRNA-9 cascade to control neural cell fate and neurogenesis. Sci Rep. 2013; 3:1329.
Article20. Wu YC, Chen CH, Mercer A, Sokol NS. Let-7-complex microRNAs regulate the temporal identity of Drosophila mushroom body neurons via chinmo. Dev Cell. 2012; 23:202–209.
Article21. Kucherenko MM, Barth J, Fiala A, Shcherbata HR. Steroid-induced microRNA let-7 acts as a spatio-temporal code for neuronal cell fate in the developing Drosophila brain. EMBO J. 2012; 31:4511–4523.
Article22. Schwamborn JC, Berezikov E, Knoblich JA. The TRIM-NHL protein TRIM32 activates microRNAs and prevents self-renewal in mouse neural progenitors. Cell. 2009; 136:913–925.
Article23. Loedige I, Filipowicz W. TRIM-NHL proteins take on miRNA regulation. Cell. 2009; 136:818–820.
Article24. Hong S, Son MR, Yun K, Lee WT, Park KA, Lee JE. Retroviral expression of human arginine decarboxylase reduces oxidative stress injury in mouse cortical astrocytes. BMC Neurosci. 2014; 15:99.
Article25. Halaris A, Plietz J. Agmatine : metabolic pathway and spectrum of activity in brain. CNS Drugs. 2007; 21:885–900.26. Yang XC, Reis DJ. Agmatine selectively blocks the N-methyl-D-aspartate subclass of glutamate receptor channels in rat hippocampal neurons. J Pharmacol Exp Ther. 1999; 288:544–549.27. Iannone L, Zhao L, Dubois O, Duluc L, Rhodes CJ, Wharton J, et al. miR-21/DDAH1 pathway regulates pulmonary vascular responses to hypoxia. Biochem J. 2014; 462:103–112.28. Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992; 116:201–211.
Article29. Fu X, Brown KJ, Yap CC, Winckler B, Jaiswal JK, Liu JS. Doublecortin (Dcx) family proteins regulate filamentous actin structure in developing neurons. J Neurosci. 2013; 33:709–721.
Article30. Shi Y, Sun G, Zhao C, Stewart R. Neural stem cell self-renewal. Crit Rev Oncol Hematol. 2008; 65:43–53.
Article31. Wang J, Wang H, Li Z, Wu Q, Lathia JD, McLendon RE, et al. c-Myc is required for maintenance of glioma cancer stem cells. PLoS One. 2008; 3:e3769.
Article32. Tocharus C, Puriboriboon Y, Junmanee T, Tocharus J, Ekthuwapranee K, Govitrapong P. Melatonin enhances adult rat hippocampal progenitor cell proliferation via ERK signaling pathway through melatonin receptor. Neuroscience. 2014; 275:314–321.
Article33. Connor B, Gordon RJ, Jones KS, Maucksch C. Deviating from the well travelled path: precursor cell migration in the pathological adult mammalian brain. J Cell Biochem. 2011; 112:1467–1474.
Article34. El-Agamy DS, Makled MN, Gamil NM. Protective effects of agmatine against D-galactosamine and lipopolysaccharide-induced fulminant hepatic failure in mice. Inflammopharmacology. 2014; 22:187–194.
Article35. Park YM, Han SH, Seo SK, Park KA, Lee WT, Lee JE. Restorative benefits of transplanting human mesenchymal stromal cells overexpressing arginine decarboxylase genes after spinal cord injury. Cytotherapy. 2015; 17:25–37.
Article36. Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005; 21:1–14.
Article37. Liu HK, Belz T, Bock D, Takacs A, Wu H, Lichter P, et al. The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes Dev. 2008; 22:2473–2478.
Article38. Wang T, Ren X, Xiong J, Zhang L, Qu J, Xu W. Tailless-like (TLX) protein promotes neuronal differentiation of dermal multipotent stem cells and benefits spinal cord injury in rats. Cell Mol Neurobiol. 2011; 31:479–487.
Article39. Kerosuo L, Piltti K, Fox H, Angers-Loustau A, Häyry V, Eilers M, et al. Myc increases self-renewal in neural progenitor cells through Miz-1. J Cell Sci. 2008; 121(Pt 23):3941–3950.
Article40. Koscianska E, Baev V, Skreka K, Oikonomaki K, Rusinov V, Tabler M, et al. Prediction and preliminary validation of oncogene regulation by miRNAs. BMC Mol Biol. 2007; 8:79.
Article41. Li Y, Liu H, Lai C, Du X, Su Z, Gao S. The Lin28/let-7a/c-Myc pathway plays a role in non-muscle invasive bladder cancer. Cell Tissue Res. 2013; 354:533–541.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Agmatine on Expression of IL-1beta and TLX Which Promotes Neuronal Differentiation in Lipopolysaccharide-Treated Neural Progenitors
- Role of agmatine in the application of neural progenitor cell in central nervous system diseases: therapeutic potentials and effects
- The Role of microRNAs Involved in Mesenchymal Stem Cell Differentiation
- Lineage-specific Expression of miR-200 Family in Human Embryonic Stem Cells during In Vitro Differentiation
- Autophagy Mediates Astrogenesis in Adult Hippocampal Neural Stem Cells