Yonsei Med J.
2016 Nov;57(6):1376-1385. 10.3349/ymj.2016.57.6.1376.
Efficacy and Safety of Infliximab Therapy and Predictors of Response in Korean Patients with Crohn's Disease: A Nationwide, Multicenter Study
- Affiliations
-
- 1Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea.
- 2Department of Internal Medicine, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Inje University College of Medicine, Busan, Korea.
- 5Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Kyungpook National University Graduate School of Medicine, Daegu, Korea.
- 7Department of Internal Medicine, Dongguk University College of Medicine, Goyang, Korea.
- 8Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea.
- 9Department of Internal Medicine, Kyung Hee University College of Medicine, Seoul, Korea.
- 10Department of Internal Medicine, Eulji University College of Medicine, Seoul, Korea.
- 11Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. kimwonho@yuhs.ac
- 12Department of Internal Medicine, Jeju National University School of Medicine, Jeju, Korea.
- 13Department of Gastroenterology, Ajou University School of Medicine, Suwon, Korea.
- 14Department of Gastroenterology, University of Ulsan College of Medicine, Seoul, Korea.
- 15Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 16Department of Internal Medicine, Konkuk University College of Medicine, Seoul, Korea.
- 17Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea.
- 18Department of Internal Medicine, Soonchunhyang University College of Medicine, Seoul, Korea.
- 19Department of Internal Medicine, Ewha Womans University School of Medicine, Seoul, Korea.
- 20Department of Internal Medicine, Hanyang University College of Medicine, Guri, Korea.
- KMID: 2427155
- DOI: http://doi.org/10.3349/ymj.2016.57.6.1376
Abstract
- PURPOSE
Infliximab is currently used for the treatment of active Crohn's disease (CD). We aimed to assess the efficacy and safety of infliximab therapy and to determine the predictors of response in Korean patients with CD.
MATERIALS AND METHODS
A total of 317 patients who received at least one infliximab infusion for active luminal CD (n=198) and fistulizing CD (n=86) or both (n=33) were reviewed retrospectively in 29 Korean referral centers. Clinical outcomes of induction and maintenance therapy with infliximab, predictors of response, and adverse events were evaluated.
RESULTS
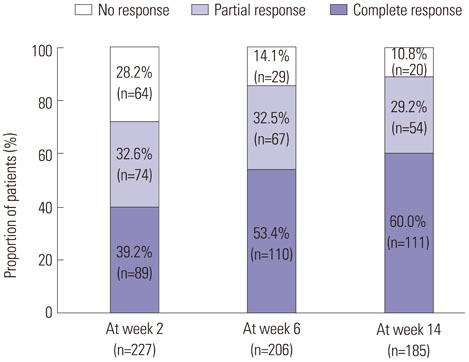
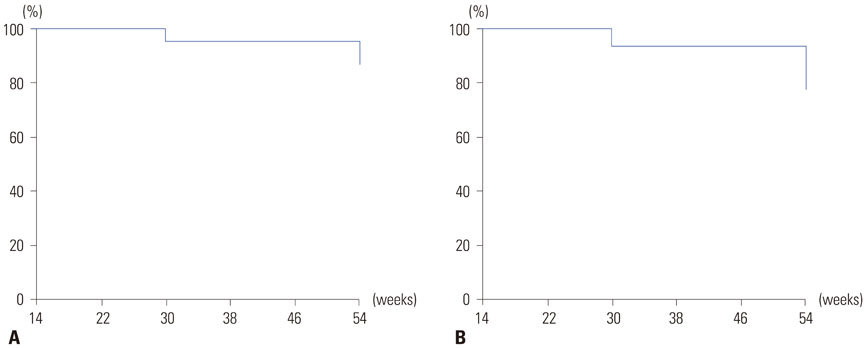
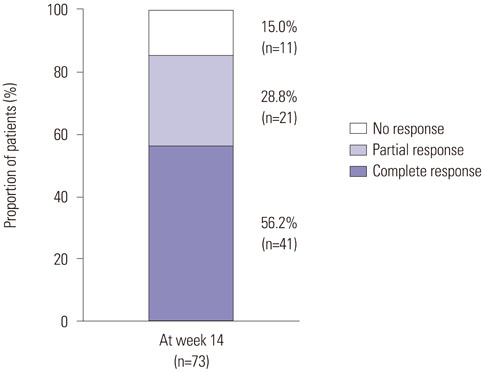
In patients with luminal CD, the rates of clinical response and remission at week 14 were 89.2% and 60.0%, respectively. Male gender and isolated colonic disease were associated with higher remission rates at week 14. In week-14 responders, the probabilities of sustained response and remission were 96.2% and 93.3% at week 30 and 88.0% and 77.0% at week 54, respectively. In patients with fistulizing CD, clinical response and remission were observed in 85.0% and 56.2% of patients, respectively, at week 14. In week-14 responders, the probabilities of sustained response and remission were 94.0% and 97.1%, respectively, at both week 30 and week 54. Thirty-nine patients (12.3%) experienced adverse events related to infliximab. Serious adverse events developed in 19 (6.0%) patients including seven cases of active pulmonary tuberculosis.
CONCLUSION
Infliximab induction and maintenance therapy are effective and well tolerable in Korean patients with luminal and fistulizing CD. However, clinicians must be aware of the risk of rare yet critical adverse events.
Keyword
MeSH Terms
-
Adolescent
Adult
Anti-Inflammatory Agents/administration & dosage
Antibodies, Monoclonal/therapeutic use
Crohn Disease/*drug therapy/ethnology
Drug Administration Schedule
Female
Gastrointestinal Agents/administration & dosage/*therapeutic use
Humans
Infliximab/*therapeutic use
Infusions, Intravenous
Male
Middle Aged
Prednisone/administration & dosage
Regression Analysis
Remission Induction
Retrospective Studies
Treatment Outcome
Anti-Inflammatory Agents
Antibodies, Monoclonal
Gastrointestinal Agents
Infliximab
Prednisone
Figure
Cited by 1 articles
-
Pathogenesis of Inflammatory Bowel Disease and Recent Advances in Biologic Therapies
Duk Hwan Kim, Jae Hee Cheon
Immune Netw. 2017;17(1):25-40. doi: 10.4110/in.2017.17.1.25.
Reference
-
1. Lichtenstein GR, Hanauer SB, Sandborn WJ. Practice Parameters Committee of American College of Gastroenterology. Management of Crohn's disease in adults. Am J Gastroenterol. 2009; 104:465–483.
Article2. Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. J Crohns Colitis. 2010; 4:7–27.
Article3. Tozer PJ, Whelan K, Phillips RK, Hart AL. Etiology of perianal Crohn's disease: role of genetic, microbiological, and immunological factors. Inflamm Bowel Dis. 2009; 15:1591–1598.
Article4. Ford AC, Kane SV, Khan KJ, Achkar JP, Talley NJ, Marshall JK, et al. Efficacy of 5-aminosalicylates in Crohn's disease: systematic review and meta-analysis. Am J Gastroenterol. 2011; 106:617–629.
Article5. Khan KJ, Dubinsky MC, Ford AC, Ullman TA, Talley NJ, Moayyedi P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011; 106:630–642.
Article6. Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007; 5:103–110.
Article7. Knight DM, Trinh H, Le J, Siegel S, Shealy D, McDonough M, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993; 30:1443–1453.
Article8. Plevy SE, Landers CJ, Prehn J, Carramanzana NM, Deem RL, Shealy D, et al. A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn's disease. J Immunol. 1997; 159:6276–6282.9. D'Haens GR, Panaccione R, Higgins PD, Vermeire S, Gassull M, Chowers Y, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn's and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011; 106:199–212.10. Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis. 2010; 4:28–62.
Article11. Hamzaoglu H, Cooper J, Alsahli M, Falchuk KR, Peppercorn MA, Farrell RJ. Safety of infliximab in Crohn's disease: a large single-center experience. Inflamm Bowel Dis. 2010; 16:2109–2116.12. Park SK, Ye BD, Lee C, Im JP, Kim YH, Kim SO, et al. Risk and clinical characteristics of lymphoma in Korean patients with inflammatory bowel diseases: a multicenter study. J Clin Gastroenterol. 2015; 49:e11–e16.13. Ng SC, Tsoi KK, Kamm MA, Xia B, Wu J, Chan FK, et al. Genetics of inflammatory bowel disease in Asia: systematic review and meta-analysis. Inflamm Bowel Dis. 2012; 18:1164–1176.
Article14. Ha C, Ullman TA, Siegel CA, Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012; 10:1002–1007.
Article15. Jo KW, Hong Y, Park JS, Bae IG, Eom JS, Lee SR, et al. Prevalence of latent tuberculosis infection among health care workers in South Korea: a multicenter study. Tuberc Respir Dis (Seoul). 2013; 75:18–24.
Article16. Lee CH, Jeong YJ, Heo EY, Park JS, Lee JS, Lee BJ, et al. Active pulmonary tuberculosis and latent tuberculosis infection among homeless people in Seoul, South Korea: a cross-sectional study. BMC Public Health. 2013; 13:720.
Article17. Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989; 170:2–6.
Article18. Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002; 359:1541–1549.
Article19. Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004; 350:876–885.
Article20. Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004; 50:1051–1065.
Article21. Ardizzone S, Colombo E, Maconi G, Bollani S, Manzionna G, Petrone MC, et al. Infliximab in treatment of Crohn's disease: the Milan experience. Dig Liver Dis. 2002; 34:411–418.
Article22. Kim YJ, Kim JW, Lee CK, Park HJ, Shim JJ, Jang JY, et al. Clinical outcome of treatment with infliximab in Crohn's disease: a single-center experience. Korean J Gastroenterol. 2013; 61:270–278.
Article23. Orlando A, Colombo E, Kohn A, Biancone L, Rizzello F, Viscido A, et al. Infliximab in the treatment of Crohn's disease: predictors of response in an Italian multicentric open study. Dig Liver Dis. 2005; 37:577–583.
Article24. Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999; 340:1398–1405.
Article25. Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease Crohn's Disease cA2 Study Group. N Engl J Med. 1997; 337:1029–1035.
Article26. Bouguen G, Levesque BG, Feagan BG, Kavanaugh A, Peyrin-Biroulet L, Colombel JF, et al. Treat to target: a proposed new paradigm for the management of Crohn's disease. Clin Gastroenterol Hepatol. 2015; 13:1042–1050.e2.
Article27. D'Haens GR, Sartor RB, Silverberg MS, Petersson J, Rutgeerts P. Future directions in inflammatory bowel disease management. J Crohns Colitis. 2014; 8:726–734.28. Peyrin-Biroulet L, Ferrante M, Magro F, Campbell S, Franchimont D, Fidder H, et al. Results from the 2nd Scientific Workshop of the ECCO I: Impact of mucosal healing on the course of inflammatory bowel disease. J Crohns Colitis. 2011; 5:477–483.
Article29. Rutgeerts P, Van Assche G, Sandborn WJ, Wolf DC, Geboes K, Colombel JF, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology. 2012; 142:1102–1111.e2.
Article30. Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007; 132:52–65.
Article31. D'Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet. 2008; 371:660–667.32. Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010; 362:1383–1395.
Article33. Centers for Disease Control and Prevention (CDC). Trends in tuberculosis--United States, 2012. MMWR Morb Mortal Wkly Rep. 2013; 62:201–205.34. Shim TS. Diagnosis and treatment of latent tuberculosis infection in patients with inflammatory bowel diseases due to initiation of anti-tumor necrosis factor therapy. Intest Res. 2014; 12:12–19.
Article35. Dixon WG, Hyrich KL, Watson KD, Lunt M, Galloway J, Ustianowski A, et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR). Ann Rheum Dis. 2010; 69:522–528.
Article36. Lee SK, Kim SY, Kim EY, Jung JY, Park MS, Kim YS, et al. Mycobacterial infections in patients treated with tumor necrosis factor antagonists in South Korea. Lung. 2013; 191:565–571.
Article37. Marehbian J, Arrighi HM, Hass S, Tian H, Sandborn WJ. Adverse events associated with common therapy regimens for moderateto-severe Crohn's disease. Am J Gastroenterol. 2009; 104:2524–2533.
Article38. Tubach F, Salmon D, Ravaud P, Allanore Y, Goupille P, Bréban M, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheum. 2009; 60:1884–1894.
Article39. Yoo IK, Choung RS, Hyun JJ, Kim SY, Jung SW, Koo JS, et al. Incidences of serious infections and tuberculosis among patients receiving anti-tumor necrosis factor-α therapy. Yonsei Med J. 2014; 55:442–448.
Article40. Joint Committee for the Revision of Korean Guidelines for Tuberculosis. Korea Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 2nd ed. Cheongju: Korea Centers for Disease Control and Prevention;2014.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adalimumab Treatment in Pediatric-Onset Crohn's Disease Patients after Infliximab Failure: A Single Center Study
- Recent Trends of Infliximab Treatment for Crohn's Disease
- Factors Affecting Surgical Treatment With Infliximab Therapy in Perianal Fistula With Crohn Disease
- Long-term Efficacy and Predictors of Response to Infliximab in Korean Patients with Crohn's Disease
- A Pityrosporum Fungal Infection Following Infliximab Therapy in a Crohn's Disease Patient