Pediatr Gastroenterol Hepatol Nutr.
2016 Jun;19(2):116-122. 10.5223/pghn.2016.19.2.116.
Adalimumab Treatment in Pediatric-Onset Crohn's Disease Patients after Infliximab Failure: A Single Center Study
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. i101016@skku.edu
- KMID: 2328172
- DOI: http://doi.org/10.5223/pghn.2016.19.2.116
Abstract
- PURPOSE
We aimed to investigate the efficacy and safety of adalimumab in pediatric-onset Crohn's disease patients who had failed treatment with infliximab.
METHODS
In this retrospective study, patients included were those who had been diagnosed with Crohn's disease before 18 years old, and had received treatment with adalimumab after infliximab failure. The efficacy of adalimumab treatment was investigated at 1 month and 1 year, and adverse events that had occurred during treatment with adalimumab were explored.
RESULTS
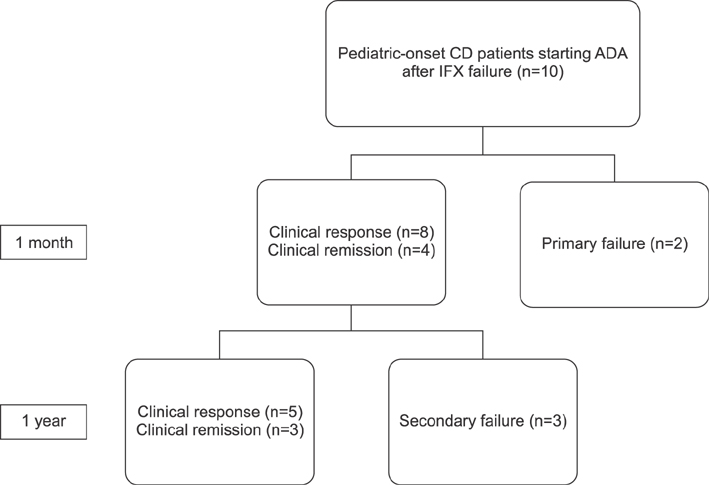
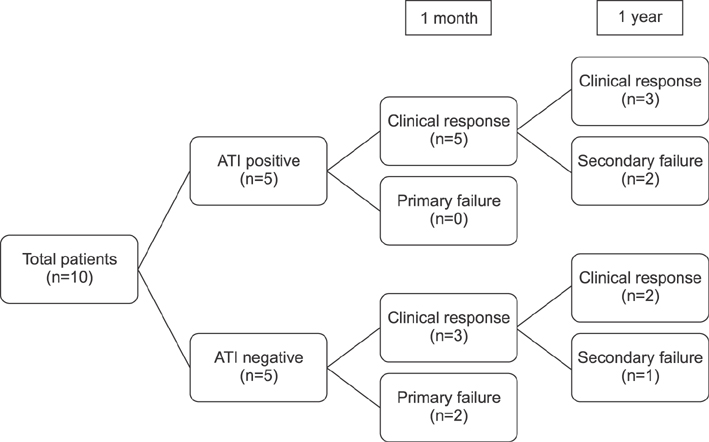
Ten patients were included in this study. The median duration from diagnosis to adalimumab treatment was 5.5 years (range: 2.4-7.9 years). At 1 month after adalimumab initiation, 80% (8/10) of patients showed clinical response, and 40% (4/10) achieved clinical remission. At 1 year, 71% (5/7) of patients showed clinical response, and 43% (3/7) were under clinical remission. Among the total included patients, 5 patients (50%) showed clinical response at 1 year. Primary non-response to adalimumab was observed in 2 patients (20%), and secondary failure to adalimumab was observed in 3 patients (30%) during 1 year treatment with adalimumab. No serious adverse event had occurred during adalimumab treatment.
CONCLUSION
Adalimumab was effective for 1 year without serious adverse events in half of pediatric-onset Crohn's disease patients who had failed treatment with infliximab.
MeSH Terms
Figure
Reference
-
1. Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007; 369:1641–1657.
Article2. Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn's disease according to the vienna classification: changing pattern over the course of the disease. Gut. 2001; 49:777–782.
Article3. Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV Jr. Risk factors associated with progression to intestinal complications of Crohn's disease in a population-based cohort. Gastroenterology. 2010; 139:1147–1155.
Article4. Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance in fliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002; 359:1541–1549.
Article5. Sands BE, Blank MA, Patel K, van Deventer SJ. ACCENT II Study. Long-term treatment of rectovaginal fistulas in Crohn's disease: response to infliximab in the ACCENT II Study. Clin Gastroenterol Hepatol. 2004; 2:912–920.
Article6. Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007; 132:863–873.
Article7. Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011; 106:685–698.
Article8. Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006; 130:323–333.
Article9. Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, et al. Adalimumab for maintenance treatment of Crohn's disease: results of the CLASSIC II trial. Gut. 2007; 56:1232–1239.
Article10. Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007; 132:52–65.
Article11. Hyams JS, Griffiths A, Markowitz J, Baldassano RN, Faubion WA Jr, Colletti RB, et al. Safety and efficacy of adalimumab for moderate to severe Crohn's disease in children. Gastroenterology. 2012; 143:365–374.e2.
Article12. Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014; 58:795–806.
Article13. Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the montreal classification for inflammatory bowel disease: the paris classification. Inflamm Bowel Dis. 2011; 17:1314–1321.
Article14. Lee YM, Kang B, Lee Y, Kim MJ, Choe YH. Infliximab "Top-Down" strategy is superior to "Step-Up" in maintaining long-term remission in the treatment of pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2015; 60:737–743.
Article15. Allez M, Karmiris K, Louis E, Van Assche G, Ben-Horin S, Klein A, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis. 2010; 4:355–366.
Article16. Rosh JR, Lerer T, Markowitz J, Goli SR, Mamula P, Noe JD, et al. Retrospective Evaluation of the Safety and Effect of Adalimumab Therapy (RESEAT) in pediatric Crohn's disease. Am J Gastroenterol. 2009; 104:3042–3049.
Article17. Russell RK, Wilson ML, Loganathan S, Bourke B, Kiparissi F, Mahdi G, et al. A british society of paediatric gastroenterology, hepatology and nutrition survey of the effectiveness and safety of adalimumab in children with inflammatory bowel disease. Aliment Pharmacol Ther. 2011; 33:946–953.
Article18. Cozijnsen M, Duif V, Kokke F, Kindermann A, van Rheenen P, de Meij T, et al. Adalimumab therapy in children with Crohn disease previously treated with infliximab. J Pediatr Gastroenterol Nutr. 2015; 60:205–210.
Article19. Fumery M, Jacob A, Sarter H, Michaud L, Spyckerelle C, Mouterde O, et al. Efficacy and safety of adalimumab after infliximab failure in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2015; 60:744–748.
Article20. Frederiksen MT, Ainsworth MA, Brynskov J, Thomsen OO, Bendtzen K, Steenholdt C. Antibodies against infliximab are associated with de novo development of antibodies to adalimumab and therapeutic failure in infliximab-to-adalimumab switchers with IBD. Inflamm Bowel Dis. 2014; 20:1714–1721.
Article21. Rutgeerts P, Van Assche G, Sandborn WJ, Wolf DC, Geboes K, Colombel JF, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology. 2012; 142:1102–1111.
Article22. Jones JL, Kaplan GG, Peyrin-Biroulet L, Baidoo L, Devlin S, Melmed GY, et al. Effects of concomitant immunomodulator therapy on efficacy and safety of anti-tumor necrosis factor therapy for Crohn's disease: a meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. 2015; 13:2233–2240.
Article23. Osterman MT, Sandborn WJ, Colombel JF, Robinson AM, Lau W, Huang B, et al. Increased risk of malignancy with adalimumab combination therapy, compared with monotherapy, for Crohn's disease. Gastroenterology. 2014; 146:941–949.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adalimumab or infliximab: which is better for perianal fistula in Crohn's disease?
- Clinical efficacy of adalimumab versus infliximab and the factors associated with recurrence or aggravation during treatment of anal fistulas in Crohn's disease
- Pulmonary Extraintestinal Manifestation of Crohn's Disease Treated Successfully with Adalimumab
- Recent Trends of Infliximab Treatment for Crohn's Disease
- Factors Affecting Surgical Treatment With Infliximab Therapy in Perianal Fistula With Crohn Disease