Investig Clin Urol.
2016 Jun;57(Suppl 1):S98-S105. 10.4111/icu.2016.57.S1.S98.
Immune checkpoint blockade therapy for bladder cancer treatment
- Affiliations
-
- 1Departments of Surgery and Biomedical Sciences, Cedars-Sinai Medical Center, Los Angeles, CA, USA. Jayoung.Kim@cshs.org
- 2Department of Medicine, University of California, Los Angeles, CA, USA.
- KMID: 2426521
- DOI: http://doi.org/10.4111/icu.2016.57.S1.S98
Abstract
- Bladder cancer remains the most immunogenic and expensive malignant tumor in the United States today. As the 4th leading cause of death from cancer in United States, Immunotherapy blocking immune checkpoints have been recently been applied to many aggressive cancers and changed interventions of urological cancers including advanced bladder cancer. The applied inhibition of PD-1-PD-L1 interactions can restore antitumor T-cell activity and enhance the cellular immune attack on antigens. The overall goals of this short review article are to introduce current cancer immunotherapy and immune checkpoint inhibitors, and to provide new insight into the underlying mechanisms that block immune checkpoints in tumor microenvironment. Furthermore, this review will address the preclinical and clinical trials to determine whether bladder cancer patients could benefit from this new cancer therapy in near future.
Keyword
MeSH Terms
-
Antibodies, Monoclonal/therapeutic use
Antineoplastic Agents/*therapeutic use
B7-H1 Antigen/antagonists & inhibitors
Humans
Immunotherapy/*methods
Molecular Targeted Therapy/methods
Programmed Cell Death 1 Receptor/antagonists & inhibitors
Urinary Bladder Neoplasms/*drug therapy/immunology
Antibodies, Monoclonal
Antineoplastic Agents
B7-H1 Antigen
Programmed Cell Death 1 Receptor
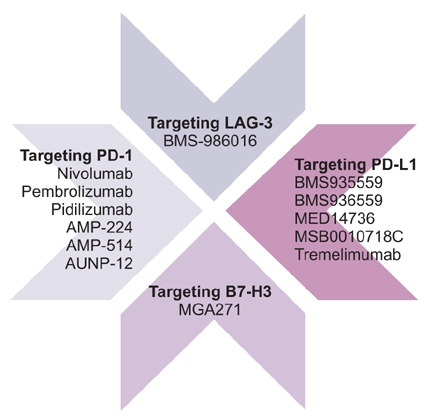
Figure
Cited by 2 articles
-
Unmasking molecular profiles of bladder cancer
Xuan-Mei Piao, Young Joon Byun, Wun-Jae Kim, Jayoung Kim
Investig Clin Urol. 2018;59(2):72-82. doi: 10.4111/icu.2018.59.2.72.Immune checkpoint inhibitors for urothelial carcinoma
Hyung Suk Kim, Ho Kyung Seo
Investig Clin Urol. 2018;59(5):285-296. doi: 10.4111/icu.2018.59.5.285.
Reference
-
1. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004; 22:329–360.2. Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012; 23:Suppl 8. viii6–viii9.3. Yuan J, Hegde PS, Clynes R, Foukas PG, Harari A, Kleen TO, et al. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J Immunother Cancer. 2016; 4:3.4. Hammerich L, Binder A, Brody JD. In situ vaccination: cancer immunotherapy both personalized and off-the-shelf. Mol Oncol. 2015; 9:1966–1981.5. Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules. Signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. 2013; 19:4917–4924.6. Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009; 374:239–249.7. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.8. Messing E. Markers of detection. Urol Oncol. 2007; 25:344–347.9. Quan C, Cha EJ, Lee HL, Han KH, Lee KM, Kim WJ. Enhanced expression of peroxiredoxin I and VI correlates with development, recurrence and progression of human bladder cancer. J Urol. 2006; 175:1512–1516.10. Bellmunt J, Pons F, Orsola A. Molecular determinants of response to cisplatin-based neoadjuvant chemotherapy. Curr Opin Urol. 2013; 23:466–471.11. Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012; 31:1869–1883.12. Lenis AT, Chamie K. Bladder cancer in 2014: from the genomic frontier to immunotherapeutics. Nat Rev Urol. 2015; 12:74–76.13. Macleod LC, Ngo TC, Gonzalgo ML. Complications of intravesical bacillus calmette-guerin. Can Urol Assoc J. 2014; 8(7-8):E540–E544.14. Steinberg RL, Thomas LJ, Nepple KG. Intravesical and alternative bladder-preservation therapies in the management of non-muscle-invasive bladder cancer unresponsive to bacillus Calmette-Guerin. Urol Oncol. 2016; Jan. 14. Epub. DOI: 10.1016/j.urolonc.2015.12.004.15. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12:252–264.16. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013; 39:1–10.17. Lesterhuis WJ, Haanen JB, Punt CJ. Cancer immunotherapy: revisited. Nat Rev Drug Discov. 2011; 10:591–600.18. Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006; 6:715–727.19. Kamat AM, Sylvester RJ, Bohle A, Palou J, Lamm DL, Brausi M, et al. Definitions, end points, and clinical trial designs for non-muscle-invasive bladder cancer: recommendations from the international bladder cancer group. J Clin Oncol. 2016; Epub. DOI: 10.1200/JCO.2015.64.4070.20. Liu S, Hou J, Zhang H, Wu Y, Hu M, Zhang L, et al. The evaluation of the risk factors for non-muscle invasive bladder cancer (NMIBC) recurrence after transurethral resection (TURBt) in Chinese population. PLoS One. 2015; 10:e0123617.21. Matsumoto K, Gondo T, Hayakawa N, Maeda T, Ninomiya A, Nakamura S. The role of single instillation chemotherapy in patients who receive subsequent bacillus Calmette-Guerin: a retrospective single centre study, and systematic review of the literature. Can Urol Assoc J. 2015; 9(7-8):E411–E416.22. O'Regan T, Tatton M, Lyon M, Masters J. The effectiveness of BCG and interferon against non-muscle invasive bladder cancer: a New Zealand perspective. BJU Int. 2015; 116:Suppl 3. 54–60.23. Ahn JJ, Ghandour RA, McKiernan JM. New agents for bacillus Calmette-Guerin-refractory nonmuscle invasive bladder cancer. Curr Opin Urol. 2014; 24:540–545.24. Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4. Clin Cancer Res. 2007; 13(18 Pt 1):5238–5242.25. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996; 271:1734–1736.26. Mokyr MB, Kalinichenko T, Gorelik L, Bluestone JA. Realization of the therapeutic potential of CTLA-4 blockade in lowdose chemotherapy-treated tumor-bearing mice. Cancer Res. 1998; 58:5301–5304.27. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patientswith metastatic melanoma. N Engl J Med. 2010; 363:711–723.28. Li Z, Chen L, Rubinstein MP. Cancer immunotherapy: are we there yet? Exp Hematol Oncol. 2013; 2:33.29. Peggs KS, Quezada SA. Ipilimumab: attenuation of an inhibitory immune checkpoint improves survival in metastatic melanoma. Expert Rev Anticancer Ther. 2010; 10:1697–1701.30. Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomized dose-comparison cohort of a phase 1 trial. Lancet. 2014; 384:1109–1117.31. Brower V. Early-stage progress on glioma vaccines. J Natl Cancer Inst. 2011; 103:1361–1362.32. Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol. 2015; 11:504–514.33. Ribas A. Clinical development of the anti-CTLA-4 antibody tremelimumab. Semin Oncol. 2010; 37:450–454.34. Kwek SS, Lewis J, Zhang L, Weinberg V, Greaney SK, Harzstark AL, et al. Preexisting levels of CD4 T cells expressing PD-1 are related to overall survival in prostate cancer patients treated with ipilimumab. Cancer Immunol Res. 2015; 3:1008–1016.35. Reese Z, Straubhar A, Pal SK, Agarwal N. Ipilimumab in the treatment of prostate cancer. Future Oncol. 2015; 11:27–37.36. Sobol I, Thompson RH, Dong H, Krco C, Kwon ED. Immunotherapy in prostate cancer. Curr Urol Rep. 2015; 16:34.37. Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007; 19:813–824.38. Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy. Inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012; 18:6580–6587.39. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002; 8:793–800.40. Seliger B, Marincola FM, Ferrone S, Abken H. The complex role of B7 molecules in tumor immunology. Trends Mol Med. 2008; 14:550–559.41. Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008; 8:467–477.42. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014; 515:568–571.43. Goldberg MV, Drake CG. LAG-3 in cancer immunotherapy. Curr Top Microbiol Immunol. 2011; 344:269–278.44. Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990; 171:1393–1405.45. Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011; 71:3540–3551.46. Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005; 6:1245–1252.47. Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007; 67:7893–7900.48. Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003; 4:899–906.49. Seliger B, Quandt D. The expression, function, and clinical relevance of B7 family members in cancer. Cancer Immunol Immunother. 2012; 61:1327–1341.50. Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A. 2003; 100:10388–10392.51. Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A. 2007; 104:19458–19463.52. Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006; 6:433–446.53. Kuespert K, Pils S, Hauck CR. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol. 2006; 18:565–571.54. Thies A, Moll I, Berger J, Wagener C, Brummer J, Schulze HJ, et al. CEACAM1 expression in cutaneous malignant melanoma predicts the development of metastatic disease. J Clin Oncol. 2002; 20:2530–2536.55. Taylor RC, Patel A, Panageas KS, Busam KJ, Brady MS. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. 2007; 25:869–875.56. Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A. 2007; 104:3967–3972.57. Fenner A. Bladder cancer: could MPDL3280A offer a therapeutic breakthrough in metastatic bladder cancer? Nat Rev Urol. 2015; 12:61.58. Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014; 515:558–562.59. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinumbased chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016; Mar. 4. Epub. DOI: 10.1016/S0140-6736(16)00561-4.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of advanced urogenital cancers with immune checkpoint inhibitors
- Advances in immune checkpoint inhibitors for hepatocellular carcinoma
- Immune Checkpoint Inhibitors in 10 Years: Contribution of Basic Research and Clinical Application in Cancer Immunotherapy
- Gut microbiome on immune checkpoint inhibitor therapy and consequent immune-related colitis: a review
- Optimising IL-2 for Cancer Immunotherapy