Intest Res.
2023 Oct;21(4):433-442. 10.5217/ir.2023.00019.
Gut microbiome on immune checkpoint inhibitor therapy and consequent immune-related colitis: a review
- Affiliations
-
- 1Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Mucosal Immunology Laboratory, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2547193
- DOI: http://doi.org/10.5217/ir.2023.00019
Abstract
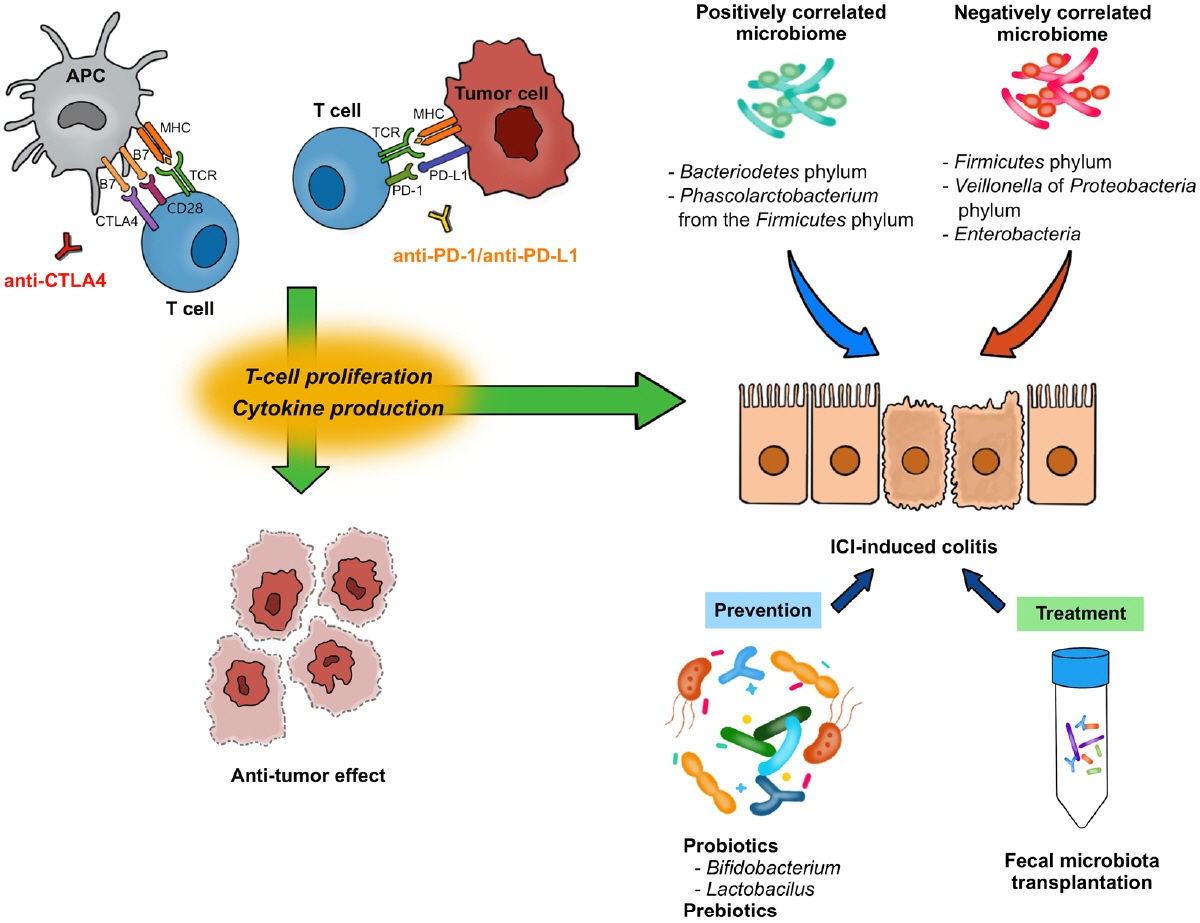
- Immune checkpoint inhibitors have dramatically revolutionized the therapeutic landscape for patients with advanced malignancies. Recently, convincing evidence has shown meaningful influence of gut microbiome on human immune system. With the complex link between gut microbiome, host immunity and cancer, the variations in the gut microbiota may influence the efficacy of immune checkpoint inhibitors. Indeed, some bacterial species have been reported to be predictive for cancer outcome in patients treated with immune checkpoint inhibitors. Although immune checkpoint inhibitors are currently proven to be an effective anti-tumor treatment, they can induce a distinct form of toxicity, termed immune-related adverse events. Immune-related colitis is one of the common toxicities from immune checkpoint inhibitors, and it might preclude the cancer therapy in severe or refractory cases. The manipulation of gut microbiome by fecal microbiota transplantation or probiotics administration has been suggested as one of the methods to enhance anti-tumor effects and decrease the risk of immune-related colitis. Here we review the role of gut microbiome on immune checkpoint inhibitor therapy and consequent immune-related colitis to provide a new insight for better anti-cancer therapy.
Keyword
Figure
Reference
-
1. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020; 11:3801.
Article2. Ephraim R, Feehan J, Fraser S, Nurgali K, Apostolopoulos V. Cancer immunotherapy: the checkpoint between chronic colitis and colorectal cancer. Cancers (Basel). 2022; 14:6131.
Article3. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010; 363:711–723.
Article4. Dobosz P, Dzieciątkowski T. The intriguing history of cancer immunotherapy. Front Immunol. 2019; 10:2965.
Article5. Jain KK. Personalized immuno-oncology. Med Princ Pract. 2021; 30:1–16.
Article6. Pennock GK, Chow LQ. The evolving role of immune checkpoint inhibitors in cancer treatment. Oncologist. 2015; 20:812–822.
Article7. Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009; 229:12–26.
Article8. Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011; 332:600–603.
Article9. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016; 39:98–106.10. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018; 8:86.
Article11. Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005; 25:9543–9553.
Article12. Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013; 342:967–970.
Article13. Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016; 535:65–74.14. Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017; 28:1368–1379.
Article15. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018; 359:97–103.16. Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018; 359:104–108.
Article17. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018; 359:91–97.18. Baruch EN, Youngster I, Ben-Betzalel G, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021; 371:602–609.
Article19. Davar D, Dzutsev AK, McCulloch JA, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021; 371:595–602.
Article20. Spencer CN, McQuade JL, Gopalakrishnan V, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021; 374:1632–1640.21. Dizman N, Meza L, Bergerot P, et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med. 2022; 28:704–712.
Article22. Zhou CB, Zhou YL, Fang JY. Gut microbiota in cancer immune response and immunotherapy. Trends Cancer. 2021; 7:647–660.
Article23. Li X, Zhang S, Guo G, Han J, Yu J. Gut microbiome in modulating immune checkpoint inhibitors. EBioMedicine. 2022; 82:104163.
Article24. Boesch M, Baty F, Albrich WC, et al. Local tumor microbial signatures and response to checkpoint blockade in non-small cell lung cancer. Oncoimmunology. 2021; 10:1988403.
Article25. Wang JW, Kuo CH, Kuo FC, et al. Fecal microbiota transplantation: review and update. J Formos Med Assoc. 2019; 118 Suppl 1:S23–S31.
Article26. Kechagia M, Basoulis D, Konstantopoulou S, et al. Health benefits of probiotics: a review. ISRN Nutr. 2013; 2013:481651.
Article27. Pinato DJ, Howlett S, Ottaviani D, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019; 5:1774–1778.
Article28. Ahmed J, Kumar A, Parikh K, et al. Use of broad-spectrum antibiotics impacts outcome in patients treated with immune checkpoint inhibitors. Oncoimmunology. 2018; 7:e1507670.
Article29. Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-smallcell lung cancer. Ann Oncol. 2018; 29:1437–1444.
Article30. Elkrief A, El Raichani L, Richard C, et al. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology. 2019; 8:e1568812.
Article31. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010; 140:805–820.
Article32. Si W, Liang H, Bugno J, et al. Lactobacillus rhamnosus GG induces cGAS/STING-dependent type I interferon and improves response to immune checkpoint blockade. Gut. 2022; 71:521–533.
Article33. Bessell CA, Isser A, Havel JJ, et al. Commensal bacteria stimulate antitumor responses via T cell cross-reactivity. JCI Insight. 2020; 5:e135597.
Article34. Overacre-Delgoffe AE, Bumgarner HJ, Cillo AR, et al. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity. 2021; 54:2812–2824.
Article35. Nomura M, Nagatomo R, Doi K, et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open. 2020; 3:e202895.
Article36. Botticelli A, Vernocchi P, Marini F, et al. Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment. J Transl Med. 2020; 18:49.
Article37. Park J, Kim M, Kang SG, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015; 8:80–93.
Article38. Mager LF, Burkhard R, Pett N, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020; 369:1481–1489.
Article39. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018; 378:158–168.
Article40. Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017; 35:785–792.
Article41. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016; 54:139–148.
Article42. Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immunerelated adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020; 6:38.
Article43. Klocke K, Sakaguchi S, Holmdahl R, Wing K. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc Natl Acad Sci U S A. 2016; 113:E2383–E2392.44. Lo B, Fritz JM, Su HC, Uzel G, Jordan MB, Lenardo MJ. CHAI and LATAIE: new genetic diseases of CTLA-4 checkpoint insufficiency. Blood. 2016; 128:1037–1042.45. Laurent S, Queirolo P, Boero S, et al. The engagement of CTLA4 on primary melanoma cell lines induces antibody-dependent cellular cytotoxicity and TNF-α production. J Transl Med. 2013; 11:108.
Article46. Tarhini AA, Zahoor H, Lin Y, et al. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer. 2015; 3:39.
Article47. Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017; 28:583–589.
Article48. Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immunecheckpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019; 16:563–580.
Article49. Nielsen DL, Juhl CB, Chen IM, Kellermann L, Nielsen OH. Immune checkpoint inhibitor-induced diarrhea and colitis: incidence and management: a systematic review and meta-analysis. Cancer Treat Rev. 2022; 109:102440.
Article50. Dougan M, Wang Y, Rubio-Tapia A, Lim JK. AGA clinical practice update on diagnosis and management of immune checkpoint inhibitor colitis and hepatitis: expert review. Gastroenterology. 2021; 160:1384–1393.
Article51. Vaziri H, Turshudzhyan A, Vecchio E. Immunotherapy-induced colitis: a comprehensive review of epidemiology, clinical presentation, diagnostic workup, and management plan. J Clin Gastroenterol. 2022; 56:555–564.52. Abu-Sbeih H, Wang Y. Management considerations for immune checkpoint inhibitor-induced enterocolitis based on management of inflammatory bowel disease. Inflamm Bowel Dis. 2020; 26:662–668.
Article53. Sakurai K, Katsurada T, Nishida M, et al. Characteristics and usefulness of transabdominal ultrasonography in immunemediated colitis. Intest Res. 2023; 21:126–136.
Article54. Kiparizoska S, Murphy ME, Mattar MC. Check this out: treatment paradigms in immune-checkpoint inhibitor colitis. Curr Opin Gastroenterol. 2023; 39:43–49.
Article55. Tan B, Liu YX, Tang H, et al. Gut microbiota shed new light on the management of immune-related adverse events. Thorac Cancer. 2022; 13:2681–2691.
Article56. Zhou G, Zhang N, Meng K, Pan F. Interaction between gut microbiota and immune checkpoint inhibitor-related colitis. Front Immunol. 2022; 13:1001623.
Article57. Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015; 350:1079–1084.
Article58. Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpointblockade-induced colitis. Nat Commun. 2016; 7:10391.
Article59. Liu T, Xiong Q, Li L, Hu Y. Intestinal microbiota predicts lung cancer patients at risk of immune-related diarrhea. Immunotherapy. 2019; 11:385–396.
Article60. Sakai K, Sakurai T, De Velasco MA, et al. Intestinal microbiota and gene expression reveal similarity and dissimilarity between immune-mediated colitis and ulcerative colitis. Front Oncol. 2021; 11:763468.
Article61. McCulloch JA, Davar D, Rodrigues RR, et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat Med. 2022; 28:545–556.
Article62. Wang F, Yin Q, Chen L, Davis MM. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc Natl Acad Sci U S A. 2018; 115:157–161.
Article63. Sun S, Luo L, Liang W, et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc Natl Acad Sci U S A. 2020; 117:27509–27515.
Article64. Wang T, Zheng N, Luo Q, et al. Probiotics Lactobacillus reuteri abrogates immune checkpoint blockade-associated colitis by inhibiting group 3 innate lymphoid cells. Front Immunol. 2019; 10:1235.
Article65. Schwartz DJ, Rebeck ON, Dantas G. Complex interactions between the microbiome and cancer immune therapy. Crit Rev Clin Lab Sci. 2019; 56:567–585.
Article66. Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013; 341:569–573.
Article67. Wang Y, Wiesnoski DH, Helmink BA, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitorassociated colitis. Nat Med. 2018; 24:1804–1808.
Article68. Fasanello MK, Robillard KT, Boland PM, Bain AJ, Kanehira K. Use of fecal microbial transplantation for immune checkpoint inhibitor colitis. ACG Case Rep J. 2020; 7:e00360.
Article69. Thompson JA, Schneider BJ, Brahmer J, et al. Management of immunotherapy-related toxicities, version 1.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022; 20:387–405.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gut microbiome and checkpoint inhibitor colitis
- Rituximab Treatment for Polyneuropathy Induced by an Immune Checkpoint Inhibitor
- Management of adverse events in cancer treatment with immune checkpoint inhibitors
- Red Blood Cell Autoantibodies in Patients Treated with Immune Checkpoint Inhibitors
- Gut-Brain Connection: Microbiome, Gut Barrier, and Environmental Sensors