Cancer Res Treat.
2018 Oct;50(4):1252-1259. 10.4143/crt.2017.438.
Phase II Study of Dovitinib in Patients with Castration-Resistant Prostate Cancer (KCSG-GU11-05)
- Affiliations
-
- 1Division of Oncology/Hematology, Department of Internal Medicine, Korea University Anam Hospital, Seoul, Korea. khpark@korea.ac.kr
- 2Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Division of Hemato-Oncology, Department of Internal Medicine, Veterans Health Service Medical Center, Seoul, Korea.
- 4Division of Hematology-Oncology, Department of Internal Medicine, Soonchunhyang University Hospital, Soonchunhyang University College of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Chungnam National University School of Medicine, Daejeon, Korea.
- 6Division of Hematology/Oncology, Department of Internal Medicine, Keimyung University Dongsan Medical Center, Daegu, Korea.
- 7Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- 8Department of Internal Medicine, Korea Cancer Center Hospital, Seoul, Korea.
- 9Dongnam Institute of Radiological and Medical Sciences, Busan, Korea.
- 10Department of Internal Medicine, Gyeongsang Institute of Health Sciences, Gyeongsang National University Changwon Hospital, Gyeongsang National University College of Medicine, Changwon, Korea.
- 11Department of Hemato-oncology, Yeungnam Medical Center, Daegu, Korea.
- 12Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2424797
- DOI: http://doi.org/10.4143/crt.2017.438
Abstract
- PURPOSE
Fibroblast growth factor (FGF) signals are important in carcinogenesis and progression of prostate cancer. Dovitinib is an oral, pan-class inhibitor of vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor, and fibroblast growth factor receptor (FGFR). We evaluated the efficacy and toxicity of dovitinib in men with metastatic castration resistant prostate cancer (mCRPC).
MATERIALS AND METHODS
This study was a single-arm, phase II, open-label, multicenter trial of dovitinib 500 mg/day (5-days-on/2-days-off schedule). The primary endpoint was 16-week progression-free survival (PFS). Secondary endpoints were overall survival (OS), toxicity and prostate-specific antigen (PSA) response rate. Biomarker analyses for VEGFR2, FGF23, and FGFR2 using multiplex enzyme-linked immunosorbent assay was performed.
RESULTS
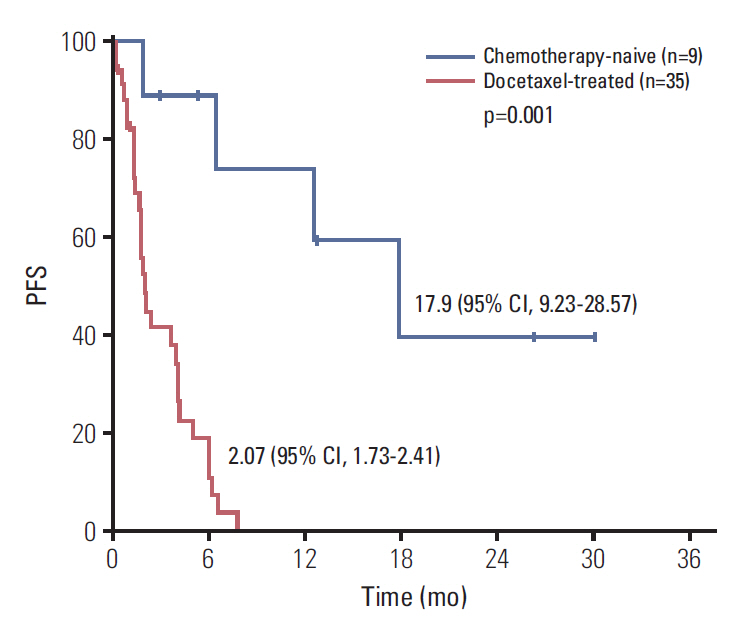
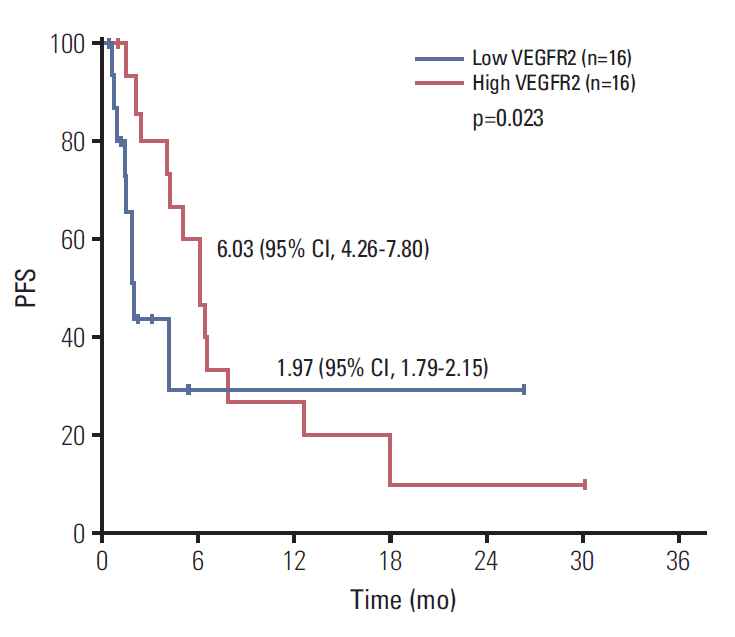
Forty-four men were accrued from 11 hospitals. Eighty percent were post-docetaxel. Median PSA was 100 ng/dL, median age was 69, 82% had bone metastases, and 23% had liver metastases. Median cycles of dovitinib was 2 (range, 0 to 33). Median PFS was 3.67 months (95% confidence interval [CI], 1.36 to 5.98) and median OS was 13.70 months (95% CI, 0 to 27.41). Chemotherapy-naïve patients had longer PFS (17.90 months; 95% CI, 9.23 to 28.57) compared with docetaxel-treated patients (2.07 months; 95% CI, 1.73 to 2.41; p=0.001) and the patients with high serum VEGFR2 level over median level (7,800 pg/mL) showed longer PFS compared with others (6.03 months [95% CI, 4.26 to 7.80] vs. 1.97 months [95% CI, 1.79 to 2.15], p=0.023). Grade 3 related adverse events were seen in 40.9% of patients. Grade 1-2 nausea, diarrhea, fatigue, anorexia, and all grade thrombocytopenia are common.
CONCLUSION
Dovitinib showed modest antitumor activity with manageable toxicities in men with mCRPC. Especially, patients who were chemo-naïve benefitted from dovitinib.
MeSH Terms
-
Anorexia
Biomarkers
Carcinogenesis
Castration
Diarrhea
Disease-Free Survival
Enzyme-Linked Immunosorbent Assay
Fatigue
Fibroblast Growth Factors
Humans
Liver
Male
Multicenter Studies as Topic
Nausea
Neoplasm Metastasis
Prostate*
Prostate-Specific Antigen
Prostatic Neoplasms*
Prostatic Neoplasms, Castration-Resistant
Receptors, Fibroblast Growth Factor
Receptors, Platelet-Derived Growth Factor
Receptors, Vascular Endothelial Growth Factor
Thrombocytopenia
Biomarkers
Fibroblast Growth Factors
Prostate-Specific Antigen
Receptors, Fibroblast Growth Factor
Receptors, Platelet-Derived Growth Factor
Receptors, Vascular Endothelial Growth Factor
Figure
Reference
-
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
Article2. Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017; 49:292–305.
Article3. Gnanapragasam VJ, Robinson MC, Marsh C, Robson CN, Hamdy FC, Leung HY. FGF8 isoform b expression in human prostate cancer. Br J Cancer. 2003; 88:1432–8.
Article4. Heer R, Douglas D, Mathers ME, Robson CN, Leung HY. Fibroblast growth factor 17 is over-expressed in human prostate cancer. J Pathol. 2004; 204:578–86.
Article5. Giri D, Ropiquet F, Ittmann M. Alterations in expression of basic fibroblast growth factor (FGF) 2 and its receptor FGFR-1 in human prostate cancer. Clin Cancer Res. 1999; 5:1063–71.6. Sahadevan K, Darby S, Leung HY, Mathers ME, Robson CN, Gnanapragasam VJ. Selective over-expression of fibroblast growth factor receptors 1 and 4 in clinical prostate cancer. J Pathol. 2007; 213:82–90.
Article7. Acevedo VD, Gangula RD, Freeman KW, Li R, Zhang Y, Wang F, et al. Inducible FGFR-1 activation leads to irreversible prostate adenocarcinoma and an epithelial-to-mesenchymal transition. Cancer Cell. 2007; 12:559–71.
Article8. Memarzadeh S, Xin L, Mulholland DJ, Mansukhani A, Wu H, Teitell MA, et al. Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer Cell. 2007; 12:572–85.
Article9. Johansson A, Rudolfsson S, Hammarsten P, Halin S, Pietras K, Jones J, et al. Mast cells are novel independent prognostic markers in prostate cancer and represent a target for therapy. Am J Pathol. 2010; 177:1031–41.
Article10. Sarker D, Molife R, Evans TR, Hardie M, Marriott C, Butzberger-Zimmerli P, et al. A phase I pharmacokinetic and pharmacodynamic study of TKI258, an oral, multitargeted receptor tyrosine kinase inhibitor in patients with advanced solid tumors. Clin Cancer Res. 2008; 14:2075–81.
Article11. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004; 351:1502–12.
Article12. de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011; 364:1995–2005.
Article13. Plosker GL. Sipuleucel-T: in metastatic castration-resistant prostate cancer. Drugs. 2011; 71:101–8.
Article14. Aragon-Ching JB, Jain L, Gulley JL, Arlen PM, Wright JJ, Steinberg SM, et al. Final analysis of a phase II trial using sorafenib for metastatic castration-resistant prostate cancer. BJU Int. 2009; 103:1636–40.
Article15. Lamont FR, Tomlinson DC, Cooper PA, Shnyder SD, Chester JD, Knowles MA. Small molecule FGF receptor inhibitors block FGFR-dependent urothelial carcinoma growth in vitro and in vivo. Br J Cancer. 2011; 104:75–82.
Article16. Armstrong K, Ahmad I, Kalna G, Tan SS, Edwards J, Robson CN, et al. Upregulated FGFR1 expression is associated with the transition of hormone-naive to castrate-resistant prostate cancer. Br J Cancer. 2011; 105:1362–9.
Article17. Angevin E, Lopez-Martin JA, Lin CC, Gschwend JE, Harzstark A, Castellano D, et al. Phase I study of dovitinib (TKI258), an oral FGFR, VEGFR, and PDGFR inhibitor, in advanced or metastatic renal cell carcinoma. Clin Cancer Res. 2013; 19:1257–68.
Article18. Motzer RJ, Porta C, Vogelzang NJ, Sternberg CN, Szczylik C, Zolnierek J, et al. Dovitinib versus sorafenib for third-line targeted treatment of patients with metastatic renal cell carcinoma: an open-label, randomised phase 3 trial. Lancet Oncol. 2014; 15:286–96.
Article19. Kim KB, Chesney J, Robinson D, Gardner H, Shi MM, Kirkwood JM. Phase I/II and pharmacodynamic study of dovitinib (TKI258), an inhibitor of fibroblast growth factor receptors and VEGF receptors, in patients with advanced melanoma. Clin Cancer Res. 2011; 17:7451–61.
Article20. Konecny GE, Finkler N, Garcia AA, Lorusso D, Lee PS, Rocconi RP, et al. Second-line dovitinib (TKI258) in patients with FGFR2-mutated or FGFR2-non-mutated advanced or metastatic endometrial cancer: a non-randomised, open-label, two-group, two-stage, phase 2 study. Lancet Oncol. 2015; 16:686–94.
Article21. Andre F, Bachelot T, Campone M, Dalenc F, Perez-Garcia JM, Hurvitz SA, et al. Targeting FGFR with dovitinib (TKI258): preclinical and clinical data in breast cancer. Clin Cancer Res. 2013; 19:3693–702.
Article22. Musolino A, Campone M, Neven P, Denduluri N, Barrios CH, Cortes J, et al. Phase II, randomized, placebo-controlled study of dovitinib in combination with fulvestrant in postmenopausal patients with HR(+), HER2(–) breast cancer that had progressed during or after prior endocrine therapy. Breast Cancer Res. 2017; 19:18.
Article23. Kang YK, Yoo C, Ryoo BY, Lee JJ, Tan E, Park I, et al. Phase II study of dovitinib in patients with metastatic and/or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib. Br J Cancer. 2013; 109:2309–15.
Article24. Laurie SA, Hao D, Leighl NB, Goffin J, Khomani A, Gupta A, et al. A phase II trial of dovitinib in previously-treated advanced pleural mesothelioma: The Ontario Clinical Oncology Group. Lung Cancer. 2017; 104:65–9.
Article25. Milowsky MI, Dittrich C, Duran I, Jagdev S, Millard FE, Sweeney CJ, et al. Phase 2 trial of dovitinib in patients with progressive FGFR3-mutated or FGFR3 wild-type advanced urothelial carcinoma. Eur J Cancer. 2014; 50:3145–52.
Article26. Musumeci M, Coppola V, Addario A, Patrizii M, Maugeri-Sacca M, Memeo L, et al. Control of tumor and microenvironment cross-talk by miR-15a and miR-16 in prostate cancer. Oncogene. 2011; 30:4231–42.
Article27. Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014; 371:424–33.
Article28. Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012; 367:1187–97.
Article29. Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013; 368:138–48.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy With Androgen Deprivation for Hormone-Naïve Prostate Cancer
- Precision Medicine in Castration-Resistant Prostate Cancer
- New Androgen Receptor Axis-Targeted Agents inthe Treatment of Nonmetastatic Castration-ResistantProstate Cancer: From Bench to Clinical Trials
- Long-Lasting Antiandrogen Withdrawal Syndrome in Castration-Resistant Prostate Cancer: Three Cases With Complete Response
- Guidelines for Evaluating Treatment Response Based on Bone Scan for Metastatic Castration-Resistant Prostate Cancer: Prostate Cancer Clinical Trial Working Group 3 Recommendations