J Vet Sci.
2018 Sep;19(5):667-675. 10.4142/jvs.2018.19.5.667.
Effect of superoxide dismutase, catalase, and glutathione peroxidase supplementation in the extender on chilled semen of fertile and hypofertile dogs
- Affiliations
-
- 1Department of Veterinary Medicine and Animal Production, University of Naples Federico II, 80137 Naples, Italy. chiara.delprete@unina.it
- 2Freelancer, 80137 Naples, Italy.
- 3Department of Biology, University of Naples Federico II, 80126 Naples, Italy.
- KMID: 2420936
- DOI: http://doi.org/10.4142/jvs.2018.19.5.667
Abstract
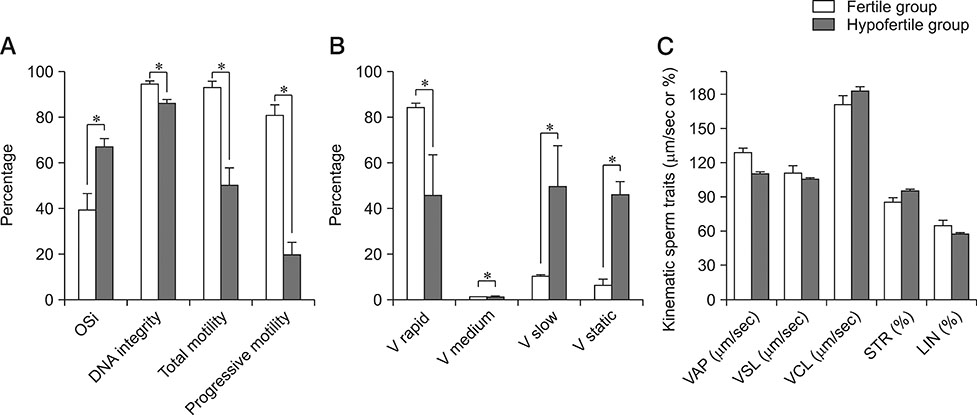
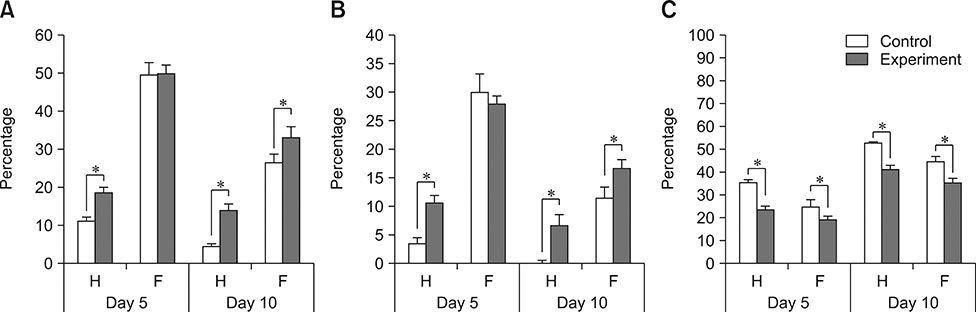
- This study investigated the correlation between oxidative stress status and key canine sperm parameters and the effect of addition of a superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) combination in egg yolk tris-citrate glucose (EYT-G) extender on semen during 10 days of storage at 4℃. Ten Boxer dogs were divided into two groups, fertile (F) and hypofertile (H), depending on pregnancy and live birth rate status in the previous year. Semen evaluation was performed on the day of collection (D0) and after 5 (D5) and 10 (D10) days of cooled storage. Sperm motility, kinetic parameters, and DNA integrity were assessed. A correlation between oxidative status and key semen parameters in both F and H groups was observed. Total and progressive motilities were significantly higher in the treated (SOD, CAT, and GPx addition) versus control groups at D10 in both F and H groups, and at D5 in the H group. DNA integrity was significantly higher in both treated groups (H and F) at D5 and D10. In conclusion, the addition of SOD, CAT, and GPx in the extender allows preservation of semen quality for up to 10 days of storage at 4℃ in both fertile and hypofertile dogs.
MeSH Terms
-
Animals
Antioxidants
Catalase*
Cats
DNA
Dogs*
Egg Yolk
Fertility
Glucose
Glutathione Peroxidase*
Glutathione*
Live Birth
Oxidative Stress
Pregnancy
Semen Analysis
Semen Preservation
Semen*
Sperm Motility
Spermatozoa
Superoxide Dismutase*
Superoxides*
Antioxidants
Catalase
DNA
Glucose
Glutathione
Glutathione Peroxidase
Superoxide Dismutase
Superoxides
Figure
Reference
-
1. Aboua YG, du Plessis SS, Reichgelt P, Brooks N. The in vitro effects of superoxide, some commercially available antioxidants and red palm oil on sperm motility. Asian J Androl. 2009; 11:695–702.
Article2. Abuelo A, Hernández J, Benedito JL, Castillo C. Oxidative stress index (OSi) as a new tool to assess redox status in dairy cattle during the transition period. Animal. 2013; 7:1374–1378.
Article3. Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003; 79:829–843.
Article4. Aitken J, Fisher H. Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. BioEssays. 1994; 16:259–267.
Article5. Aitken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod. 1989; 41:183–197.
Article6. Aurich JE, Schönherr U, Hoppe H, Aurich C. Effects of antioxidants on motility and membrane integrity of chilled-stored stallion semen. Theriogenology. 1997; 48:185–192.
Article7. Beconi MT, Francia CR, Mora NG, Affranchino MA. Effect of natural antioxidants on frozen bovine semen preservation. Theriogenology. 1993; 40:841–851.
Article8. Boni R, Cocchia N, Silvestre F, Tortora G, Lorizio R, Tosti E. Juvenile and adult immature and in vitro matured ovine oocytes evaluated in relation to membrane electrical properties, calcium stores, IP3 sensitivity and apoptosis occurrence in cumulus cells. Mol Reprod Dev. 2008; 75:1752–1760.
Article9. Bouchard GF, Morris JK, Sikes JD, Youngquist RS. Effect of storage temperature, cooling rates and two different semen extenders on canine spermatozoal motility. Theriogenology. 1990; 34:147–157.
Article10. Cesarone MR, Belcaro G, Carratelli M, Cornelli U. A simple test to monitor oxidative stress. Int Angiol. 1999; 18:127–130.11. Chatdarong K, Chaivechakarn A, Thuwanut P, Ponglowhapan S. Effects of cold storage prior to freezing on superoxide dismutase, glutathione peroxidase activities, level of total reactive oxygen species and sperm quality in dogs. Reprod Domest Anim. 2012; 47:Suppl 6. 274–277.
Article12. Chen SJ, Allam JP, Duan YG, Haidl G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet. 2013; 288:191–199.
Article13. Cocchia N, Pasolini MP, Mancini R, Petrazzuolo O, Cristofaro I, Rosapane I, Sica A, Tortora G, Lorizio R, Paraggio G, Mancini A. Effect of sod (superoxide dismutase) protein supplementation in semen extenders on motility, viability, acrosome status and ERK (extracellular signal-regulated kinase) protein phosphorylation of chilled stallion spermatozoa. Theriogenology. 2011; 75:1201–1210.
Article14. Cocuzza M, Sikka SC, Athayde KS, Agarwal A. Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: an evidence based analysis. Int Braz J Urol. 2007; 33:603–621.
Article15. De Iuliis GN, Thomson LK, Mitchell LA, Finnie JM, Koppers AJ, Hedges A, Nixon B, Aitken RJ. DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2′-deoxyguanosine, a marker of oxidative stress. Biol Reprod. 2009; 81:517–524.
Article16. Griveau JF, Le Lannou D. Reactive oxygen species and human spermatozoa: physiology and pathology. Int J Androl. 1997; 20:61–69.
Article17. Hirano Y, Shibahara H, Obara H, Suzuki T, Takamizawa S, Yamaguchi C, Tsunoda H, Sato I. Relationships between sperm motility characteristics assessed by the computer-aided sperm analysis (CASA) and fertilization rates in vitro. J Assist Reprod Genet. 2001; 18:213–218.18. Hori T, Ichikawa M, Kawakami E, Tsutsui T. Artificial insemination of frozen epididymal sperm in beagle dogs. J Vet Med Sci. 2004; 66:37–41.
Article19. Hu JH, Tian WQ, Zhao XL, Zan LS, Wang H, Li QW, Xin YP. The cryoprotective effects of ascorbic acid supplementation on bovine semen quality. Anim Reprod Sci. 2010; 121:72–77.
Article20. Jarosz Ł, Grądzki Z, Kalinowski M, Laskowska E. Quality of fresh and chilled-stored raccoon dog semen and its impact on artificial insemination efficiency. BMC Vet Res. 2016; 12:224.
Article21. Jewgenow K, Songsasen N. Reproduction and advances in reproductive studies in carnivores. In : Holt WV, Brown JL, Comizzoli P, editors. Reproductive Sciences in Animal Conservation. New York: Springer;2014. p. 205–239.22. Kasimanickam VR, Kasimanickam RK, Memon MA, Rogers HA. Effect of extenders on sperm mitochondrial membrane, plasma membrane and sperm kinetics during liquid storage of canine semen at 5℃. Anim Reprod Sci. 2012; 136:139–145.
Article23. Kawakami E, Takemura A, Sakuma M, Takano M, Hirano T, Hori T, Tsutsui T. Superoxide dismutase and catalase activities in the seminal plasma of normozoospermic and asthenozoospermic Beagles. J Vet Med Sci. 2007; 69:133–136.
Article24. Kumi-Diaka J, Badtram G. Effect of storage on sperm membrane integrity and other functional characteristics of canine spermatozoa: in vitro bioassay for canine semen. Theriogenology. 1994; 41:1355–1366.
Article25. Kutluyer F, Kayim M, Öğretmen F, Büyükleblebici S, Tuncer PB. Cryopreservation of rainbow trout Oncorhynchus mykiss spermatozoa: effects of extender supplemented with different antioxidants on sperm motility, velocity and fertility. Cryobiology. 2014; 69:462–466.
Article26. Lopes-Santiago BV, Monteiro GA, Bittencourt R, Arduino F, Ovidio PP, Jordão-Junior AA, Araùjo JP Jr, Lopes MD. Evaluation of sperm DNA peroxidation in fertile and subfertile dogs. Reprod Domest Anim. 2012; 47:Suppl 6. 208–209.
Article27. Luberda Z. [Present conception regarding the effect on reactive oxygen species on functions of mammalian spermatozoa]. Postępy Biologii Komórki. 2001; 28:309–316. Polish.28. Martin G, Sabido O, Durand P, Levy R. Cryopreservation induces an apoptosis-like mechanism in bull sperm. Biol Reprod. 2004; 71:28–37.
Article29. Michael AJ, Alexopoulos C, Pontiki EA, Hadjipavlou-Litina DJ, Saratsis P, Ververidis HN, Boscos CM. Quality and reactive oxygen species of extended canine semen after vitamin C supplementation. Theriogenology. 2008; 70:827–835.
Article30. Neagu VR, García BM, Rodríguez AM, Ferrusola CO, Bolaños JM, Fernández LG, Tapia JA, Peña FJ. Determination of glutation peroxidase and superoxide dismutase activities in canine seminal plasma and its relation with sperm quality and lipid peroxidation post thaw. Theriogenology. 2011; 75:10–16.
Article31. Ortega-Ferrusola C, Sotillo-Galán Y, Varela-Fernández E, Gallardo-Bolaños JM, Muriel A, González-Fernández L, Tapia JA, Peña FJ. Detection of “apoptosis-like” changes during the cryopreservation process in equine sperm. J Androl. 2008; 29:213–221.
Article32. Perrin A, Basinko A, Douet-Guilbert N, Gueganic N, Le Bris MJ, Amice V, De Braekeleer M, Morel F. Aneuploidy and DNA fragmentation in sperm of carriers of a constitutional chromosomal abnormality. Cytogenet Genome Res. 2011; 133:100–106.
Article33. Ponglowhapan S, Essén-Gustavsson B, Linde Forsberg C. Influence of glucose and fructose in the extender during long-term storage of chilled canine semen. Theriogenology. 2004; 62:1498–1517.
Article34. Province CA, Amann RP, Pickett BW, Squires EL. Extenders for preservation of canine and equine spermatozoa at 5℃. Theriogenology. 1984; 22:409–415.
Article35. Rota A, Ström B, Linde-Forsberg C. Effects of seminal plasma and three extenders on canine semen stored at 4℃. Theriogenology. 1995; 44:885–900.
Article36. Said TM, Gaglani A, Agarwal A. Implication of apoptosis in sperm cryoinjury. Reprod Biomed Online. 2010; 21:456–462.
Article37. Storey BT. Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Mol Hum Reprod. 1997; 3:203–213.
Article38. Strzezek R, Koziorowska-Gilun M, Kowalówka M, Strzezek J. Characteristics of antioxidant system in dog semen. Pol J Vet Sci. 2009; 12:55–60.39. Tapia JA, Macias-Garcia B, Miro-Moran A, Ortega-Ferrusola C, Salido GM, Peña FJ, Aparicio IM. The membrane of the mammalian spermatozoa: much more than an inert envelope. Reprod Domest Anim. 2012; 47:Suppl 3. 65–75.
Article40. Tsutsui T, Tezuka T, Mikasa Y, Sugisawa H, Kirihara N, Hori T, Kawakami E. Artificial insemination with canine semen stored at a low temperature. J Vet Med Sci. 2003; 65:307–312.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The activities of antioxidant enzymes in erythrocytes of newborn infants
- Activities of scavenging enzymes of oxygen radicals in early maturation stages of Paragonimus westermani
- Activities of Hepatic Antioxidant Enzymes in Bile Duct Ligates Rats
- Effect of Hemodialysis on Levels of Malondialdehyde and Antioxidant Enzymes in Erythrocytes from Patients with End Stage Renal Disease
- Superoxide Dismutase, Catalase and Glutathione Peroxidase Activities in Erythrocytes and Synovial Fluid of the Osteoarthritis of the Knee Joint