J Vet Sci.
2018 Sep;19(5):592-599. 10.4142/jvs.2018.19.5.592.
In vitro differentiation of spermatogonial stem cells using testicular cells from Guangxi Bama mini-pig
- Affiliations
-
- 1State Key Laboratory for Conservation and Utilization of Subtropical Agro-bioresources, Guangxi High Education Key Laboratory for Animal Reproduction and Biotechnology, College of Animal Science and Technology, Guangxi University, Nanning 530005, China. shengshenglu@sina.com
- 2College of Life Science and Technology, Guangxi University, Nanning 530005, China.
- KMID: 2420927
- DOI: http://doi.org/10.4142/jvs.2018.19.5.592
Abstract
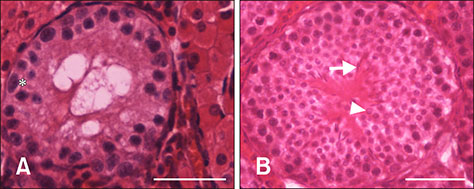
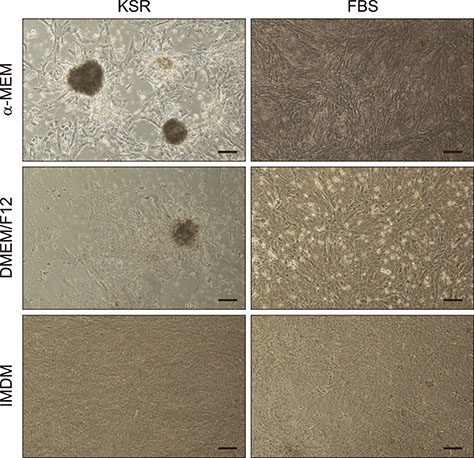
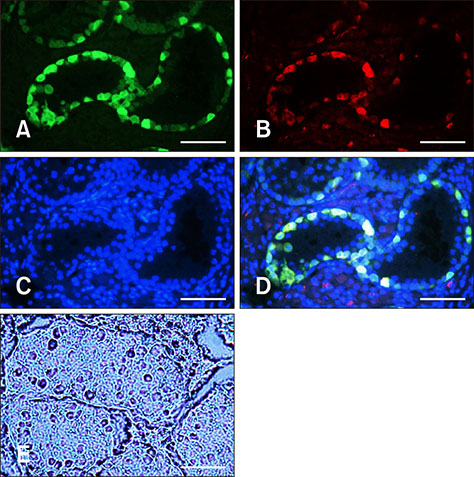
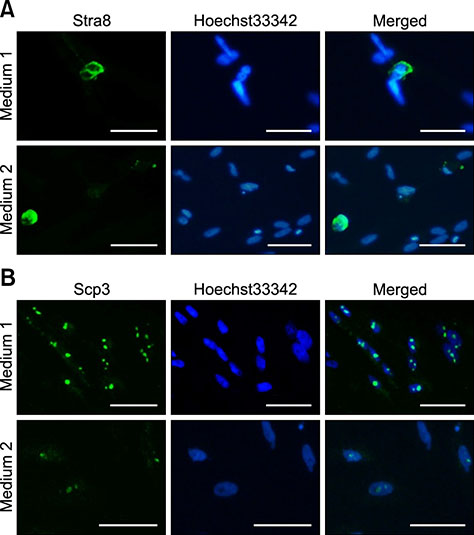
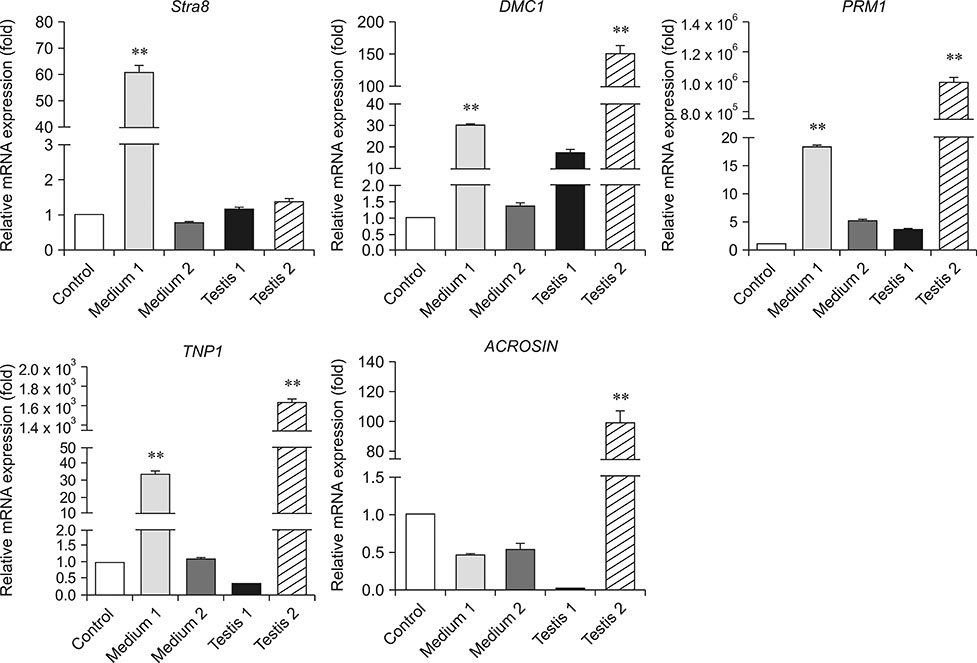
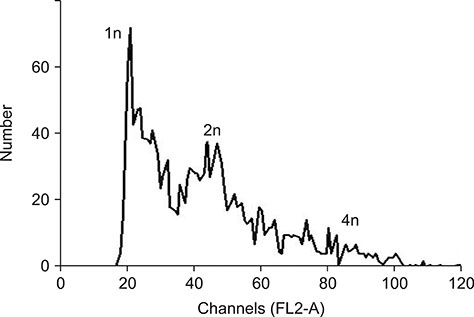
- In this study, we attempted to establish a culture system for in vitro spermatogenesis from spermatogonial stem cells (SSCs) of Bama mini-pig. Dissociated testicular cells from 1-month-old pigs were co-cultured to mimic in vivo spermatogenesis. The testicular cells were seeded in minimum essential medium alpha (α-MEM) supplemented with Knockout serum replacement (KSR). Three-dimensional colonies formed after 10 days of culture. The colonies showed positive staining for SSC-associated markers such as UCHL1, PLZF, THY1, OCT4, Dolichos biflorus agglutinin, and alkaline phosphatase. Induction of SSCs was performed in α-MEM + KSR supplemented with retinoic acid, bone morphogenetic protein 4, activin A, follicle-stimulating hormone, or testosterone. The results showed that STRA8, DMC1, PRM1, and TNP1 were upregulated significantly in the colonies after induction compared to that in testis from 1-month-old pigs, while expression levels of those genes were significantly low compared to those in 2-month-old testis. However, upregulation of ACROSIN was not significant. Replacement of α-MEM and KSR with Iscove's modified Dulbecco's medium and fetal bovine serum did not upregulate expression of these genes significantly. These results indicate that SSCs of Bama mini-pig could undergo differentiation and develop to a post-meiotic stage in α-MEM supplemented with KSR and induction factors.
Keyword
MeSH Terms
-
Acrosin
Activins
Alkaline Phosphatase
Bone Morphogenetic Protein 4
Dolichos
Follicle Stimulating Hormone
Humans
In Vitro Techniques*
Infant
Infant, Newborn
Spermatogenesis
Stem Cells*
Swine
Testis
Testosterone
Tretinoin
Up-Regulation
Acrosin
Activins
Alkaline Phosphatase
Bone Morphogenetic Protein 4
Follicle Stimulating Hormone
Testosterone
Tretinoin
Figure
Cited by 1 articles
-
Derivation of endothelial cells from porcine induced pluripotent stem cells by optimized single layer culture system
Renyue Wei, Jiawei Lv, Xuechun Li, Yan Li, Qianqian Xu, Junxue Jin, Yu Zhang, Zhonghua Liu
J Vet Sci. 2020;21(1):. doi: 10.4142/jvs.2020.21.e9.
Reference
-
1. Abu Elhija M, Lunenfeld E, Schlatt S, Huleihel M. Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian J Androl. 2012; 14:285–293.
Article2. Akbarinejad V, Tajik P, Movahedin M, Youssefi R, Shafiei S, Mazaheri Z. Effect of extracellular matrix on bovine spermatogonial stem cells and gene expression of niche factors regulating their development in vitro. Anim Reprod Sci. 2015; 157:95–102.
Article3. Aoshima K, Baba A, Makino Y, Okada Y. Establishment of alternative culture method for spermatogonial stem cells using knockout serum replacement. PLoS One. 2013; 8:e77715.
Article4. Baazm M, Abolhassani F, Abbasi M, Habibi Roudkenar M, Amidi F, Beyer C. An improved protocol for isolation and culturing of mouse spermatogonial stem cells. Cell Reprogram. 2013; 15:329–336.
Article5. Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science. 2006; 312:596–600.
Article6. Bukovsky A, Caudle MR, Gupta SK, Svetlikova M, Selleck-White R, Ayala AM, Dominguez R. Mammalian neo-oogenesis and expression of meiosis-specific protein SCP3 in adult human and monkey ovaries. Cell Cycle. 2008; 7:683–686.
Article7. Deng S, Wang X, Wang Z, Chen S, Wang Y, Hao X, Sun T, Zhang Y, Lian Z, Liu Y. In vitro production of functional haploid sperm cells from male germ cells of Saanen dairy goat. Theriogenology. 2017; 90:120–128.
Article8. Erkkilä K, Henriksén K, Hirvonen V, Rannikko S, Salo J, Parvinen M, Dunkel L. Testosterone regulates apoptosis in adult human seminiferous tubules in vitro. J Clin Endocrinol Metab. 1997; 82:2314–2321.
Article9. Feng LX, Chen Y, Dettin L, Pera RA, Herr JC, Goldberg E, Dym M. Generation and in vitro differentiation of a spermatogonial cell line. Science. 2002; 297:392–395.
Article10. Ferrer M, Rodriguez H, Zara L, Yu Y, Xu W, Oko R. MMP2 and acrosin are major proteinases associated with the inner acrosomal membrane and may cooperate in sperm penetration of the zona pellucida during fertilization. Cell Tissue Res. 2012; 349:881–895.
Article11. Fujihara M, Kim SM, Minami N, Yamada M, Imai H. Characterization and in vitro culture of male germ cells from developing bovine testis. J Reprod Dev. 2011; 57:355–364.
Article12. Goel S, Sugimoto M, Minami N, Yamada M, Kume S, Imai H. Identification, isolation, and in vitro culture of porcine gonocytes. Biol Reprod. 2007; 77:127–137.13. Heidari B, Rahmati-Ahmadabadi M, Akhondi MM, Zarnani AH, Jeddi-Tehrani M, Shirazi A, Naderi MM, Behzadi B. Isolation, identification, and culture of goat spermatogonial stem cells using c-kit and PGP9.5 markers. J Assist Reprod Genet. 2012; 29:1029–1038.
Article14. Hu J, Chen YX, Wang D, Qi X, Li TG, Hao J, Mishina Y, Garbers DL, Zhao GQ. Developmental expression and function of Bmp4 in spermatogenesis and in maintaining epididymal integrity. Dev Biol. 2004; 276:158–171.
Article15. Izadyar F, Den Ouden K, Creemers LB, Posthuma G, Parvinen M, De Rooij DG. Proliferation and differentiation of bovine type A spermatogonia during long-term culture. Biol Reprod. 2003; 68:272–281.
Article16. Kanatsu-Shinohara M, Ogonuki N, Matoba S, Morimoto H, Ogura A, Shinohara T. Improved serum- and feeder-free culture of mouse germline stem cells. Biol Reprod. 2014; 91:88.17. Kuijk EW, Colenbrander B, Roelen BA. The effects of growth factors on in vitro-cultured porcine testicular cells. Reproduction. 2009; 138:721–731.
Article18. Lee JH, Kim HJ, Kim H, Lee SJ, Gye MC. In vitro spermatogenesis by three-dimensional culture of rat testicular cells in collagen gel matrix. Biomaterials. 2006; 27:2845–2853.
Article19. Mithraprabhu S, Mendis S, Meachem SJ, Tubino L, Matzuk MM, Brown CW, Loveland KL. Activin bioactivity affects germ cell differentiation in the postnatal mouse testis in vivo. Biol Reprod. 2010; 82:980–990.
Article20. Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, Kubota Y, Ogawa T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011; 471:504–507.
Article21. Takeda N, Yoshinaga K, Furushima K, Takamune K, Li Z, Abe S, Aizawa S, Yamamura K. Viable offspring obtained from Prm1-deficient sperm in mice. Sci Rep. 2016; 6:27409.
Article22. Tesarik J, Greco E, Mendoza C. Assisted reproduction with in-vitro-cultured testicular spermatozoa in cases of severe germ cell apoptosis: a pilot study. Hum Reprod. 2001; 16:2640–2645.
Article23. Tiptanavattana N, Techakumphu M, Tharasanit T. Simplified isolation and enrichment of spermatogonial stem-like cells from pubertal domestic cats (Felis catus). J Vet Med Sci. 2015; 77:1347–1353.
Article24. Wang H, Sun M, Qin Y, Xia T, Ma J, Chen ZJ. Mutations in DMC1 are not responsible for premature ovarian failure in Chinese women. Reprod Biomed Online. 2013; 26:175–178.
Article25. West FD, Mumaw JL, Gallegos-Cardenas A, Young A, Stice SL. Human haploid cells differentiated from meiotic competent clonal germ cell lines that originated from embryonic stem cells. Stem Cells Dev. 2011; 20:1079–1088.
Article26. Yang S, Ping P, Ma M, Li P, Tian R, Yang H, Liu Y, Gong Y, Zhang Z, Li Z, He Z. Generation of haploid spermatids with fertilization and development capacity from human spermatogonial stem cells of cryptorchid patients. Stem Cell Reports. 2014; 3:663–675.
Article27. Yu YE, Zhang Y, Unni E, Shirley CR, Deng JM, Russell LD, Weil MM, Behringer RR, Meistrich ML. Abnormal spermatogenesis and reduced fertility in transition nuclear protein 1-deficient mice. Proc Nat Acad Sci USA. 2000; 97:4683–4688.
Article28. Zhao HM, Yang H, Luo FH, Li MX, Zhang S, Yang XG, Lu YQ, Lu SS, Wu YJ, Lu KH. Isolation, proliferation, and induction of Bama mini-pig spermatogonial stem cells in vitro. Genet Mol Res. 2016; 15:15038602.
Article29. Zhou Q, Nie R, Li Y, Friel P, Mitchell D, Hess RA, Small C, Griswold MD. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol Reprod. 2008; 79:35–42.
Article30. Zhou Q, Wang M, Yuan Y, Wang X, Fu R, Wan H, Xie M, Liu M, Guo X, Zheng Y, Feng G, Shi Q, Zhao XY, Sha J, Zhou Q. Complete meiosis from embryonic stem cell-derived germ cells in vitro. Cell Stem Cell. 2016; 18:330–340.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dimethyloxaloylglycine promotes spermatogenesis activity of spermatogonial stem cells in Bama minipigs
- Evaluating the effect of conditioned medium from mesenchymal stem cells on differentiation of rat spermatogonial stem cells
- Effects of different culture systems on the culture of prepuberal buffalo (Bubalus bubalis) spermatogonial stem cell-like cells in vitro
- Inhibition of Class I Histone Deacetylase Enhances Self-Reprogramming of Spermatogonial Stem Cells into Pluripotent Stem Cells
- Adaptation of Human Testicular Niche Cells for Pluripotent Stem Cell and Testis Development Research