Ann Dermatol.
2018 Jun;30(3):322-330. 10.5021/ad.2018.30.3.322.
Investigation of Immune-Regulatory Effects of Mageumsan Hot Spring via Protein Microarray In Vitro
- Affiliations
-
- 1Department of Dermatology, Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Uijeongbu, Korea. jwkim52@catholic.ac.kr
- KMID: 2419174
- DOI: http://doi.org/10.5021/ad.2018.30.3.322
Abstract
- BACKGROUND
Empirical evidences for efficacy of hot spring (HS) water in inflammatory skin disorders have not been substantiated with sufficient, immunological "hard evidence". Mageumsan HS water, characterized by its weakly-alkaline properties and low total dissolved solids content, has been known to alleviate various immune-inflammatory skin diseases, including atopic dermatitis (AD).
OBJECTIVE
The trial attempted to quantitatively analyze in vitro expression levels of chemical mediators in cutaneous inflammation from HaCaT cell line treated with Mageumsan HS, and suggest the likely mode of action through which it exerts the apparent anti-inflammatory effects in AD.
METHODS
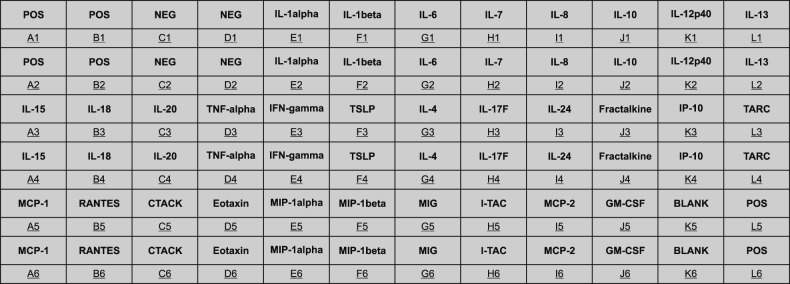
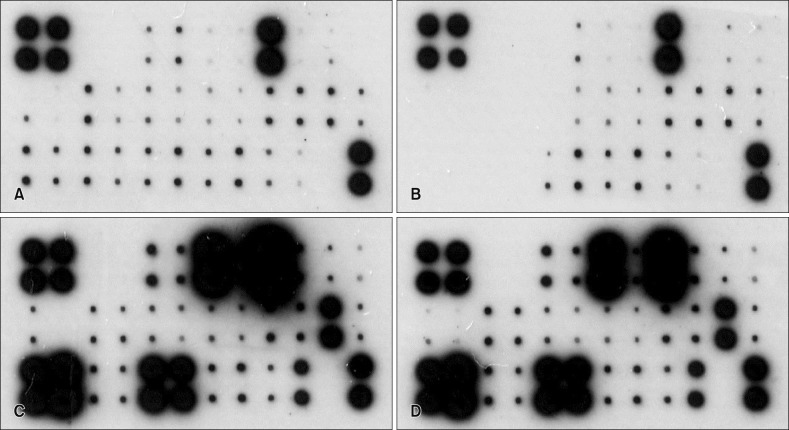
Using membrane-based human antibody array kit, customized to include 30 different, keratinocyte-derived mediator proteins, their expression levels (including interleukin [IL]-1, IL-6, IL-8, thymic stromal lymphopoietin, thymus and activation-regulated chemokine, and granulocyte macrophage colony-stimulating factor) were assessed in vitro. Selected key proteins were further quantified with enzyme-linked immunosorbent assay.
RESULTS
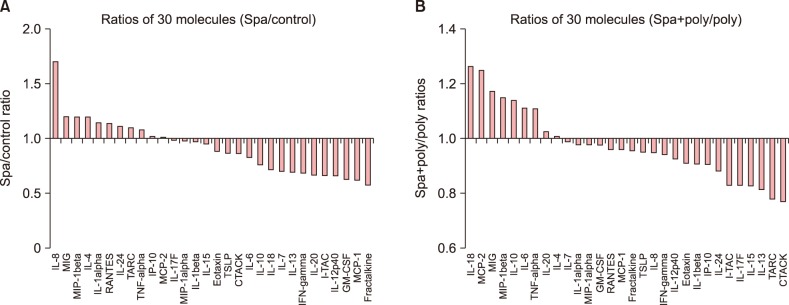
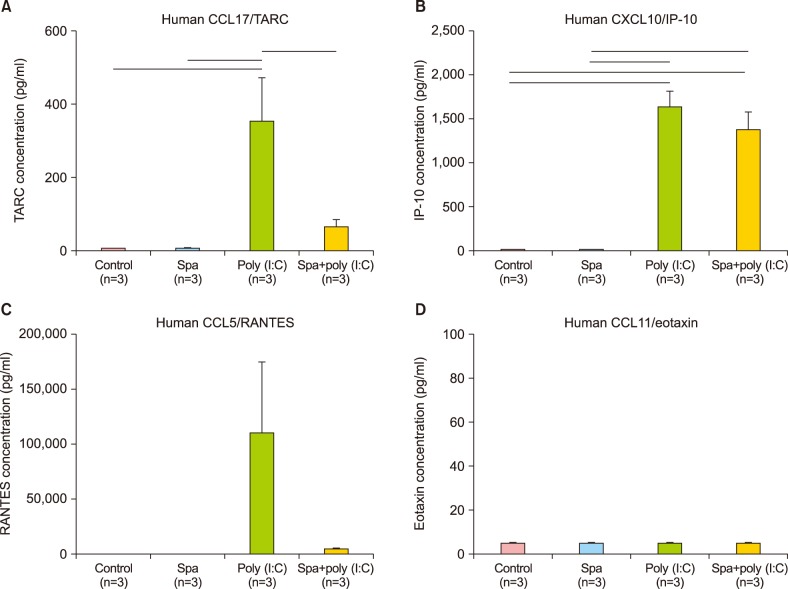
There was a clear pattern of overall suppression of the mediators, especially those noted for their pro-inflammatory role in AD (monocyte chemoattractant protein [MCP]-1, regulated on activation, normal T cell expressed and secreted, cutaneous T-cell-attracting chemokine, Eotaxin, and macrophage inflammatory protein-1α, etc.). Also, reduced expression of involucrin and cytokeratin 1 was also reduced in the HS-treated group.
CONCLUSION
The present study has shown that Mageumsan HS water may exert its effects on inflammatory skin disorders through regulation of proinflammatory cytokines. These evidences are to be supported with further future investigations to elucidate immunological mechanism behind these beneficial effects of HS water in the chronically inflamed skin of AD.
Keyword
MeSH Terms
-
Cell Line
Chemokine CCL17
Chemokine CCL27
Cytokines
Dermatitis, Atopic
Enzyme-Linked Immunosorbent Assay
Granulocytes
Hot Springs*
Humans
In Vitro Techniques*
Inflammation
Interleukin-6
Interleukin-8
Interleukins
Keratins
Macrophages
Protein Array Analysis*
Skin
Skin Diseases
Water
Chemokine CCL17
Chemokine CCL27
Cytokines
Interleukin-6
Interleukin-8
Interleukins
Keratins
Water
Figure
Reference
-
1. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003; 16:132–140. PMID: 12919115.
Article2. Kim JW. Therapeutic effectiveness and its underlying immunologic mechanisms of Korean hot spring water on atopic dermatitis. J Korean Acad Hot Spring. 2012; 1:48–54.3. Kim KH, Yun ST, Park SS, Choi BY, Chang ES, Ko YG. Physicochemical characteristics of natural hot spring waters in Korea and geochemical modeling of water quality change in heating-cooling. J Korean Acad Hot Spring. 2012; 1:22–33.4. Kim JW, Hahn HJ, Woo SY, Yun ST, Lee JT, Kim HJ. Immunoinflammatory regulation effects of Korean hot spring water. J Jpn Soc Balneol Climatol Phys Med. 2015; 78:253–270.5. Lee HP, Choi YJ, Cho KA, Woo SY, Yun ST, Lee JT, et al. Effect of spa spring water on cytokine expression in human keratinocyte HaCaT cells and on differentiation of CD4(+) T cells. Ann Dermatol. 2012; 24:324–336. PMID: 22879717.6. Choi YJ, Lee HJ, Lee DH, Woo SY, Lee KH, Yun ST, et al. Therapeutic effects and immunomodulation of suanbo mineral water therapy in a murine model of atopic dermatitis. Ann Dermatol. 2013; 25:462–470. PMID: 24371394.
Article7. Lee KH, Cho KA, Kim JY, Kim JY, Baek JH, Woo SY, et al. Filaggrin knockdown and Toll-like receptor 3 (TLR3) stimulation enhanced the production of thymic stromal lymphopoietin (TSLP) from epidermal layers. Exp Dermatol. 2011; 20:149–151. PMID: 21255094.
Article8. Lee YB, Lee JY, Lee HJ, Yun ST, Lee JT, Kim HJ, et al. Immunomodulatory effects of balneotherapy with hae-un-dae thermal water on imiquimod-induced psoriasis-like murine model. Ann Dermatol. 2014; 26:221–230. PMID: 24882978.
Article9. Irvine AD, Eichenfield LF, Friedlander SF, Simpson EL. Review of critical issues in the pathogenesis of atopic dermatitis. Semin Cutan Med Surg. 2016; 35(5 Suppl):S89–S91. PMID: 27525507.
Article10. Simpson EL, Irvine AD, Eichenfield LF, Friedlander SF. Update on epidemiology, diagnosis, and disease course of atopic dermatitis. Semin Cutan Med Surg. 2016; 35(5 Suppl):S84–S88. PMID: 27525380.11. Sinke JD, Rutten VP, Willemse T. Immune dysregulation in atopic dermatitis. Vet Immunol Immunopathol. 2002; 87:351–356. PMID: 12072258.
Article12. Ong PY, Leung DY. Immune dysregulation in atopic dermatitis. Curr Allergy Asthma Rep. 2006; 6:384–389. PMID: 16899200.
Article13. Ou LS, Goleva E, Hall C, Leung DY. T regulatory cells in atopic dermatitis and subversion of their activity by superantigens. J Allergy Clin Immunol. 2004; 113:756–763. PMID: 15100684.
Article14. Werfel T, Biedermann T. Current novel approaches in systemic therapy of atopic dermatitis: specific inhibition of cutaneous Th2 polarized inflammation and itch. Curr Opin Allergy Clin Immunol. 2015; 15:446–452. PMID: 26308331.15. Seltmann J, Werfel T, Wittmann M. Evidence for a regulatory loop between IFN-γ and IL-33 in skin inflammation. Exp Dermatol. 2013; 22:102–107. PMID: 23362867.
Article16. Knol EF, Hijnen D. Atopic dermatitis: a tale of two distinct pathomechanisms that make you itch. Eur J Immunol. 2016; 46:2512–2515. PMID: 27813070.
Article17. Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-tohigh-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016; 388:31–44. PMID: 27130691.18. Glowacka E, Lewkowicz P, Rotsztejn H, Zalewska A. IL-8, IL-12 and IL-10 cytokines generation by neutrophils, fibroblasts and neutrophils- fibroblasts interaction in psoriasis. Adv Med Sci. 2010; 55:254–260. PMID: 20934961.
Article19. Bobbala D, Mayhue M, Menendez A, Ilangumaran S, Ramanathan S. Trans-presentation of interleukin-15 by interleukin-15 receptor alpha is dispensable for the pathogenesis of autoimmune type 1 diabetes. Cell Mol Immunol. 2017; 14:590–596. PMID: 26853723.
Article20. Jin SH, Choi D, Chun YJ, Noh M. Keratinocyte-derived IL-24 plays a role in the positive feedback regulation of epidermal inflammation in response to environmental and endogenous toxic stressors. Toxicol Appl Pharmacol. 2014; 280:199–206. PMID: 25168428.
Article21. Strbo N, Yin N, Stojadinovic O. Innate and adaptive immune responses in wound epithelialization. Adv Wound Care (New Rochelle). 2014; 3:492–501. PMID: 25032069.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Anti-inflammatory and Antimicrobial Effects of Selenium-rich Hot Spring Water on a Chronic Bacterial Prostatitis Rat Model
- The Effect of Selenium-rich Hot Spring Water on Serum Leptin and IGF-1 in a Rat Model
- Regulatory T Cells in B Cell Follicles
- Effect of Spa Spring Water on Cytokine Expression in Human Keratinocyte HaCaT Cells and on Differentiation of CD4+ T Cells
- Effect of an 8-week hot spring water exercise program on body composition, knee isokinetic muscle function, range of motion, and the Korean Western Ontario and Mcmaster Universities index in middle-age females with osteoarthritis in Republic of Korea: experiment study