Korean J Radiol.
2018 Oct;19(5):888-896. 10.3348/kjr.2018.19.5.888.
Effect of Hybrid Kernel and Iterative Reconstruction on Objective and Subjective Analysis of Lung Nodule Calcification in Low-Dose Chest CT
- Affiliations
-
- 1Department of Radiology, College of Medicine, Dong-A University, Busan 49201, Korea. medcarrot@dau.ac.kr
- 2Department of Radiology, College of Medicine, Kyungpook National University, Daegu 41944, Korea.
- KMID: 2418552
- DOI: http://doi.org/10.3348/kjr.2018.19.5.888
Abstract
OBJECTIVE
To evaluate the differences in subjective calcification detection rates and objective calcium volumes in lung nodules according to different reconstruction methods using hybrid kernel (FC13-H) and iterative reconstruction (IR).
MATERIALS AND METHODS
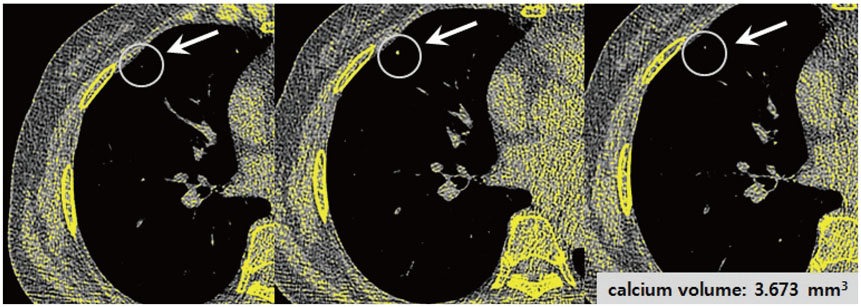
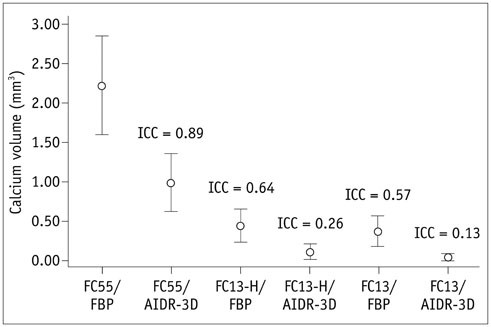
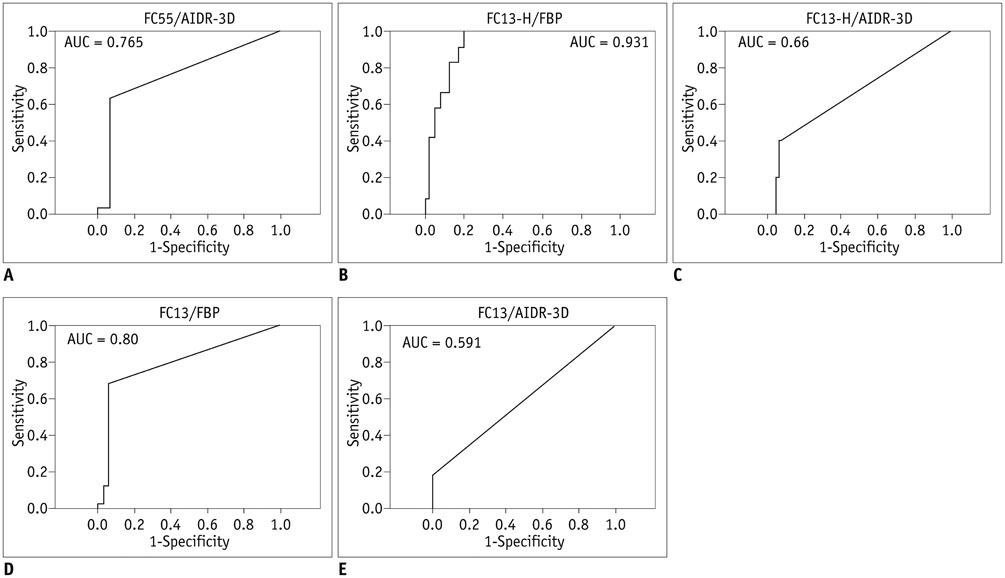
Overall, 35 patients with small (< 4 mm) calcified pulmonary nodules on chest CT were included. Raw data were reconstructed using filtered back projection (FBP) or IR algorithm (AIDR-3D; Canon Medical Systems Corporation), with three types of reconstruction kernel: conventional lung kernel (FC55), FC13-H and conventional soft tissue kernel (FC13). The calcium volumes of pulmonary nodules were quantified using the modified Agatston scoring method. Two radiologists independently interpreted the role of each nodule calcification on the six types of reconstructed images (FC55/FBP, FC55/AIDR-3D, FC13-H/FBP, FC13-H/AIDR-3D, FC13/FBP, and FC13/AIDR-3D).
RESULTS
Seventy-eight calcified nodules detected on FC55/FBP images were regarded as reference standards. The calcium detection rates of FC55/AIDR-3D, FC13-H/FBP, FC13-H/AIDR-3D, FC13/FBP, and FC13/AIDR-3D protocols were 80.7%, 15.4%, 6.4%, 52.6%, and 28.2%, respectively, and FC13-H/AIDR-3D showed the smallest calcium detection rate. The calcium volume varied significantly with reconstruction protocols and FC13/AIDR-3D showed the smallest calcium volume (0.04 ± 0.22 mm³), followed by FC13-H/AIDR-3D.
CONCLUSION
Hybrid kernel and IR influence subjective detection and objective measurement of calcium in lung nodules, particularly when both techniques (FC13-H/AIDR-3D) are combined.
Figure
Reference
-
1. Chiles C. Lung cancer screening with low-dose computed tomography. Radiol Clin North Am. 2014; 52:27–46.
Article2. Kalra MK, Maher MM, Toth TL, Hamberg LM, Blake MA, Shepard JA, et al. Strategies for CT radiation dose optimization. Radiology. 2004; 230:619–628.
Article3. Kim Y, Kim YK, Lee BE, Lee SJ, Ryu YJ, Lee JH, et al. Ultra-low-dose CT of the thorax using iterative reconstruction: evaluation of image quality and radiation dose reduction. AJR Am J Roentgenol. 2015; 204:1197–1202.
Article4. Singh S, Kalra MK, Gilman MD, Hsieh J, Pien HH, Digumarthy SR, et al. Adaptive statistical iterative reconstruction technique for radiation dose reduction in chest CT: a pilot study. Radiology. 2011; 259:565–573.
Article5. Katsura M, Matsuda I, Akahane M, Sato J, Akai H, Yasaka K, et al. Model-based iterative reconstruction technique for radiation dose reduction in chest CT: comparison with the adaptive statistical iterative reconstruction technique. Eur Radiol. 2012; 22:1613–1623.
Article6. Lim HJ, Chung MJ, Shin KE, Hwang HS, Lee KS. The impact of iterative reconstruction in low-dose computed tomography on the evaluation of diffuse interstitial lung disease. Korean J Radiol. 2016; 17:950–960.
Article7. Padole A, Ali Khawaja RD, Kalra MK, Singh S. CT radiation dose and iterative reconstruction techniques. AJR Am J Roentgenol. 2015; 204:W384–W392.
Article8. Grewal RG, Austin JH. CT demonstration of calcification in carcinoma of the lung. J Comput Assist Tomogr. 1994; 18:867–871.
Article9. Fischbach F, Knollmann F, Griesshaber V, Freund T, Akkol E, Felix R. Detection of pulmonary nodules by multislice computed tomography: improved detection rate with reduced slice thickness. Eur Radiol. 2003; 13:2378–2383.
Article10. Sinsuat M, Saita S, Kawata Y, Niki N, Ohmatsu H, Tsuchida T, et al. Influence of slice thickness on diagnoses of pulmonary nodules using low-dose CT: potential dependence of detection and diagnostic agreement on features and location of nodule. Acad Radiol. 2011; 18:594–604.11. National Lung Screening Trial Research Team. Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013; 368:1980–1991.
Article12. Kazerooni EA, Austin JH, Black WC, Dyer DS, Hazelton TR, Leung AN, et al. American College of Radiology. Society of Thoracic Radiology. ACR-STR practice parameter for the performance and reporting of lung cancer screening thoracic computed tomography (CT): 2014 (Resolution 4). J Thorac Imaging. 2014; 29:310–331.13. Rubin GD. Data explosion: the challenge of multidetector-row CT. Eur J Radiol. 2000; 36:74–80.
Article14. Strub WM, Weiss KL, Sun D. Hybrid reconstruction kernel: optimized chest CT. AJR Am J Roentgenol. 2007; 189:W115–W116.
Article15. Khan AN, Al-Jahdali HH, Allen CM, Irion KL, Al Ghanem S, Koteyar SS. The calcified lung nodule: what does it mean? Ann Thorac Med. 2010; 5:67–79.
Article16. Toshiba Aquilion ONE Operation Manual 2B201-417EN*P. Toshiba Scanner TSX-301A. Toshiba Medical System Cooperation. 2011. p. 167–214. Chapter 12 eXam Plan Management.17. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990; 15:827–832.
Article18. Christner JA, Kofler JM, McCollough CH. Estimating effective dose for CT using dose-length product compared with using organ doses: consequences of adopting International Commission on Radiological Protection publication 103 or dual-energy scanning. AJR Am J Roentgenol. 2010; 194:881–889.
Article19. Webb WR. Radiologic evaluation of the solitary pulmonary nodule. AJR Am J Roentgenol. 1990; 154:701–708.
Article20. Diederich S, Wormanns D, Heindel W. Lung cancer screening with low-dose CT. Eur J Radiol. 2003; 45:2–7.
Article21. Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999; 354:99–105.
Article22. Swensen SJ, Jett JR, Hartman TE, Midthun DE, Sloan JA, Sykes AM, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology. 2003; 226:756–761.
Article23. Wormanns D, Kohl G, Klotz E, Marheine A, Beyer F, Heindel W, et al. Volumetric measurements of pulmonary nodules at multi-row detector CT: in vivo reproducibility. Eur Radiol. 2004; 14:86–92.
Article24. Armato SG 3rd, Altman MB, La Rivière PJ. Automated detection of lung nodules in CT scans: effect of image reconstruction algorithm. Med Phys. 2003; 30:461–472.
Article25. Willemink MJ, Leiner T, de Jong PA, de Heer LM, Nievelstein RA, Schilham AM, et al. Iterative reconstruction techniques for computed tomography part 2: initial results in dose reduction and image quality. Eur Radiol. 2013; 23:1632–1642.
Article26. Schindler A, Vliegenthart R, Schoepf UJ, Blanke P, Ebersberger U, Cho YJ, et al. Iterative image reconstruction techniques for CT coronary artery calcium quantification: comparison with traditional filtered back projection in vitro and in vivo. Radiology. 2014; 270:387–393.
Article27. van Osch JA, Mouden M, van Dalen JA, Timmer JR, Reiffers S, Knollema S, et al. Influence of iterative image reconstruction on CT-based calcium score measurements. Int J Cardiovasc Imaging. 2014; 30:961–967.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Deep Learning-Based Reconstruction Algorithm With Lung Enhancement Filter for Chest CT: Effect on Image Quality and Ground Glass Nodule Sharpness
- Performance of Half-dose Chest Computed Tomography in Lung Malignancy Using an Iterative Reconstruction Technique
- Dosimetric Effects of Low Dose 4D CT Using a Commercial Iterative Reconstruction on Dose Calculation in Radiation Treatment Planning: A Phantom Study
- Contrast-Enhanced CT with Knowledge-Based Iterative Model Reconstruction for the Evaluation of Parotid Gland Tumors: A Feasibility Study
- Radiation Dose Reduction of Chest CT with Iterative Reconstruction in Image Space - Part I: Studies on Image Quality Using Dual Source CT