Intest Res.
2018 Apr;16(2):233-245. 10.5217/ir.2018.16.2.233.
Tofacitinib induction and maintenance therapy in East Asian patients with active ulcerative colitis: subgroup analyses from three phase 3 multinational studies
- Affiliations
-
- 1IBD Center, Sapporo Kosei General Hospital, Sapporo, Japan.
- 2Department of Gastroenterology and Hepatology, Tokyo Medical and Dental University, Tokyo, Japan.
- 3Division of Gastroenterology, Department of Internal Medicine, Kyung Hee University School of Medicine, Seoul, Korea.
- 4Division of Gastroenterology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Hanyang University Guri Hospital, Guri, Korea.
- 6Inflammation & Immunology, Pfizer Japan Inc, Tokyo, Japan.
- 7Center for Advanced IBD Research and Treatment, Kitasato University, Tokyo, Japan. thibi@insti.kitasato-u.ac.jp
- KMID: 2417675
- DOI: http://doi.org/10.5217/ir.2018.16.2.233
Abstract
- BACKGROUND/AIMS
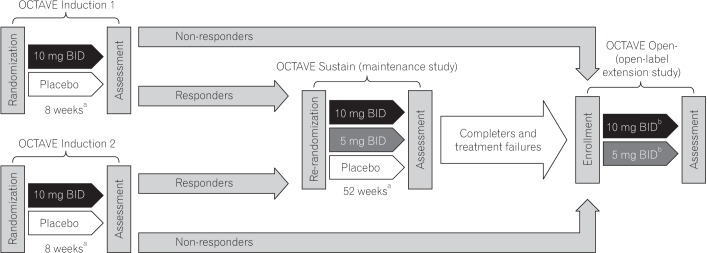
Tofacitinib is an oral, small-molecule Janus kinase inhibitor being investigated for ulcerative colitis (UC). In OCTAVE Induction 1 and 2, patients with moderately to severely active UC received placebo or tofacitinib 10 mg twice daily (BID) for 8 weeks. Clinical responders in OCTAVE Induction were re-randomized to 52 weeks' therapy with placebo, tofacitinib 5 mg BID, or tofacitinib 10 mg BID.
METHODS
We conducted post-hoc efficacy and safety analyses of East Asian patients in OCTAVE Induction 1 and 2 and OCTAVE Sustain.
RESULTS
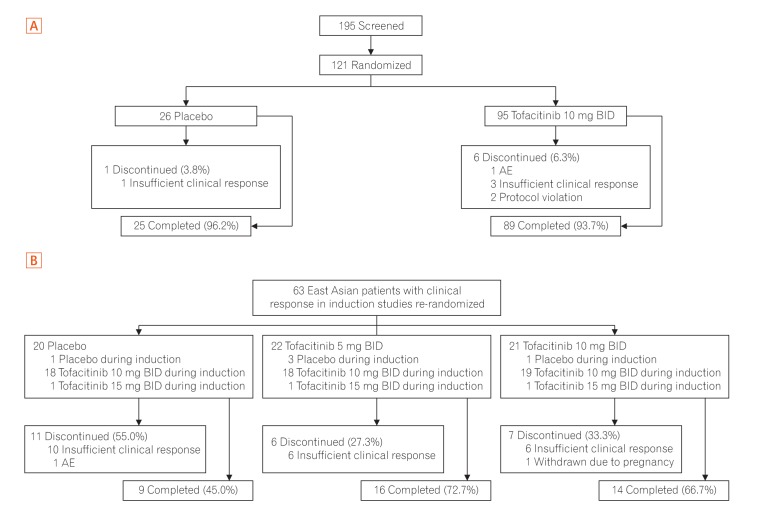
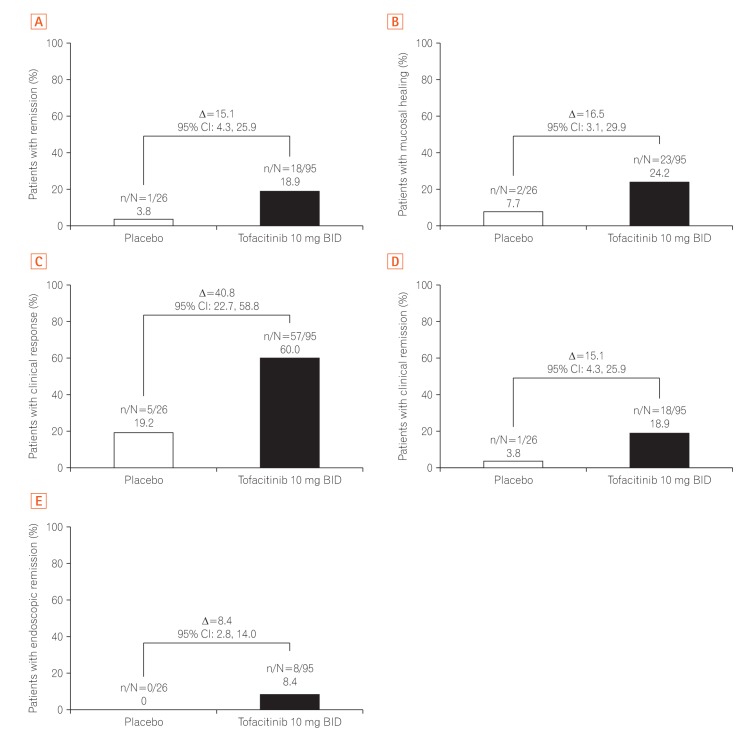
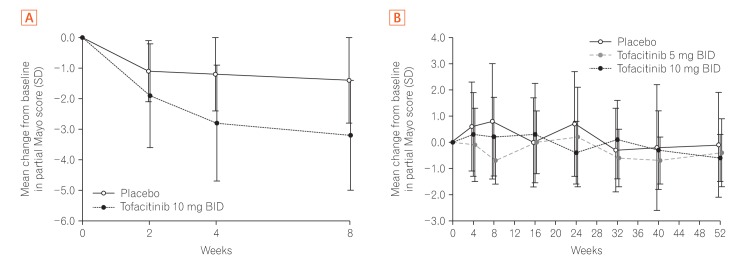
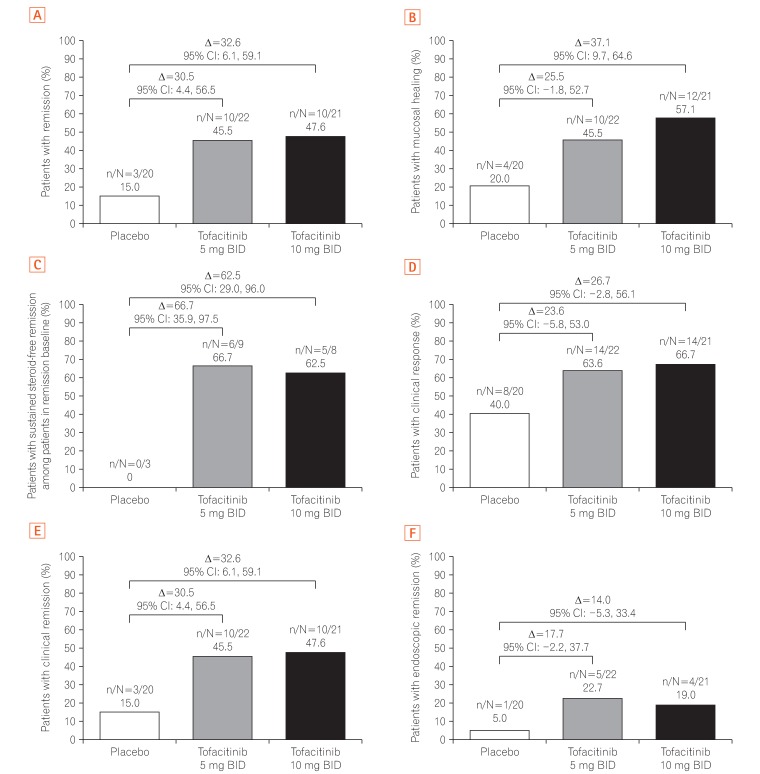
A total of 121 East Asian (Japan, Korea, and Taiwan) patients were randomized in OCTAVE Induction 1 and 2 (placebo, n=26; tofacitinib 10 mg BID, n=95), and 63 in OCTAVE Sustain (placebo, n=20; tofacitinib 5 mg BID, n=22; tofacitinib 10 mg BID, n=21). At week 8 of OCTAVE Induction 1 and 2, 18.9% of patients (18/95) achieved remission with tofacitinib 10 mg BID versus 3.8% (1/26) with placebo. In OCTAVE Sustain, the week 52 remission rates were 45.5% (10/22), 47.6% (10/21), and 15.0% (3/20) with 5 mg BID, 10 mg BID, and placebo, respectively. Adverse event rates were similar between groups in OCTAVE Induction and numerically higher with tofacitinib in OCTAVE Sustain. Serious adverse event rates were similar across groups in all studies. Infections were numerically more frequent with tofacitinib than placebo. Increases in serum lipid levels were observed with tofacitinib.
CONCLUSIONS
In East Asian patients with UC, tofacitinib demonstrated numerically greater efficacy versus placebo as induction and maintenance therapy, with a safety profile consistent with the global study population. ClinicalTrials.gov: NCT01465763; NCT01458951; NCT01458574.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Efficacy of biologic therapies for biologic-naïve Japanese patients with moderately to severely active ulcerative colitis: a network meta-analysis
Toshifumi Hibi, Isao Kamae, Philippe Pinton, Lyann Ursos, Ryuichi Iwakiri, Greg Hather, Haridarshan Patel
Intest Res. 2021;19(1):53-61. doi: 10.5217/ir.2019.09146.Efficacy and safety of ustekinumab in East Asian patients with moderately to severely active ulcerative colitis: a subpopulation analysis of global phase 3 induction and maintenance studies (UNIFI)
Tadakazu Hisamatsu, Hyo Jong Kim, Satoshi Motoya, Yasuo Suzuki, Yoshifumi Ohnishi, Noriyuki Fujii, Nobuko Matsushima, Richuan Zheng, Colleen W. Marano
Intest Res. 2021;19(4):386-397. doi: 10.5217/ir.2020.00080.
Reference
-
1. Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012; 380:1606–1619. PMID: 22914296.
Article2. Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: a comparison with developed countries and regional differences. J Dig Dis. 2010; 11:134–147. PMID: 20579217.
Article3. Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986–2005: a KASID study. Inflamm Bowel Dis. 2008; 14:542–549. PMID: 17941073.
Article4. Asakura K, Nishiwaki Y, Inoue N, Hibi T, Watanabe M, Takebayashi T. Prevalence of ulcerative colitis and Crohn's disease in Japan. J Gastroenterol. 2009; 44:659–665. PMID: 19424654.
Article5. Ouyang Q, Tandon R, Goh KL, Ooi CJ, Ogata H, Fiocchi C. The emergence of inflammatory bowel disease in the Asian Pacific region. Curr Opin Gastroenterol. 2005; 21:408–413. PMID: 15930979.6. Chen CY, Lee KT, Tzu-Chi LC, Lai WT, Huang YB. Epidemiology and disease burden of ulcerative colitis in Taiwan: a nationwide population-based study. Value Health Reg Issues. 2013; 2:127–134. PMID: 29702840.
Article7. Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016; 14:111–119. PMID: 27175111.
Article8. Hu PJ. Inflammatory bowel disease in Asia: the challenges and opportunities. Intest Res. 2015; 13:188–190. PMID: 26130991.
Article9. Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012; 367:616–624. PMID: 22894574.
Article10. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017; 376:1723–1736. PMID: 28467869.
Article11. Irvine EJ, Feagan B, Rochon J, et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn’s Relapse Prevention Trial Study Group. Gastroenterology. 1994; 106:287–296. PMID: 8299896.
Article12. Wollenhaupt J, Silverfield J, Lee EB, et al. Safety and efficacy of tofacitinib, an oral janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol. 2014; 41:837–852. PMID: 24692527.
Article13. Papp KA, Krueger JG, Feldman SR, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: long-term efficacy and safety results from 2 randomized phase-III studies and 1 open-label long-term extension study. J Am Acad Dermatol. 2016; 74:841–850. PMID: 26899199.
Article14. Sandborn WJ, Ghosh S, Panes J, et al. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2014; 12:1485–1493.e2. PMID: 24480677.15. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; 353:2462–2476. PMID: 16339095.
Article16. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012; 142:257–265.e3. PMID: 22062358.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corrigendum: Tofacitinib induction and maintenance therapy in East Asian patients with active ulcerative colitis: subgroup analyses from three phase 3 multinational studies
- Efficacy and safety of ustekinumab in East Asian patients with moderately to severely active ulcerative colitis: a subpopulation analysis of global phase 3 induction and maintenance studies (UNIFI)
- Effects of Dextran Sulfate Sodium-Induced Ulcerative Colitis on the Disposition of Tofacitinib in Rats
- Efficacy and safety of vedolizumab in ulcerative colitis in patients from Asian countries in the GEMINI 1 study
- Long-term efficacy and safety of tofacitinib in patients with ulcerative colitis: 3-year results from a real-world study