Efficacy and safety of ustekinumab in East Asian patients with moderately to severely active ulcerative colitis: a subpopulation analysis of global phase 3 induction and maintenance studies (UNIFI)
- Affiliations
-
- 1Department of Gastroenterology and Hepatology, Kyorin University School of Medicine, Tokyo, Japan
- 2Center for Crohn’s and Colitis, Department of Gastroenterology, Kyung Hee University College of Medicine, Seoul, Korea
- 3IBD Center, Hokkaido Prefectural Welfare Federation of Agricultural Cooperative, Sapporo-Kosei General, Hokkaido, Japan
- 4Division of Gastroenterology and Hepatology, Department of Internal Medicine, Toho University Sakura Medical Center, Sakura, Japan
- 5Division of Gastroenterology, Shizuoka Medical Center, Shizuoka, Japan
- 6Janssen Pharmaceutical K.K., Tokyo, Japan
- 7Janssen Research & Development, LLC, Spring House, PA, USA
- KMID: 2521602
- DOI: http://doi.org/10.5217/ir.2020.00080
Abstract
- Background/Aims
We aimed to evaluate the efficacy and safety of ustekinumab (UST) in the East-Asian population with moderate to severely active ulcerative colitis (UC).
Methods
This sub-analysis was conducted on data from East-Asian patients included in the UNIFI program (NCT02407236). UNIFI consisted of two double-blind, placebo-controlled trials: an 8-week induction study and a 44-week randomized withdrawal maintenance study.
Results
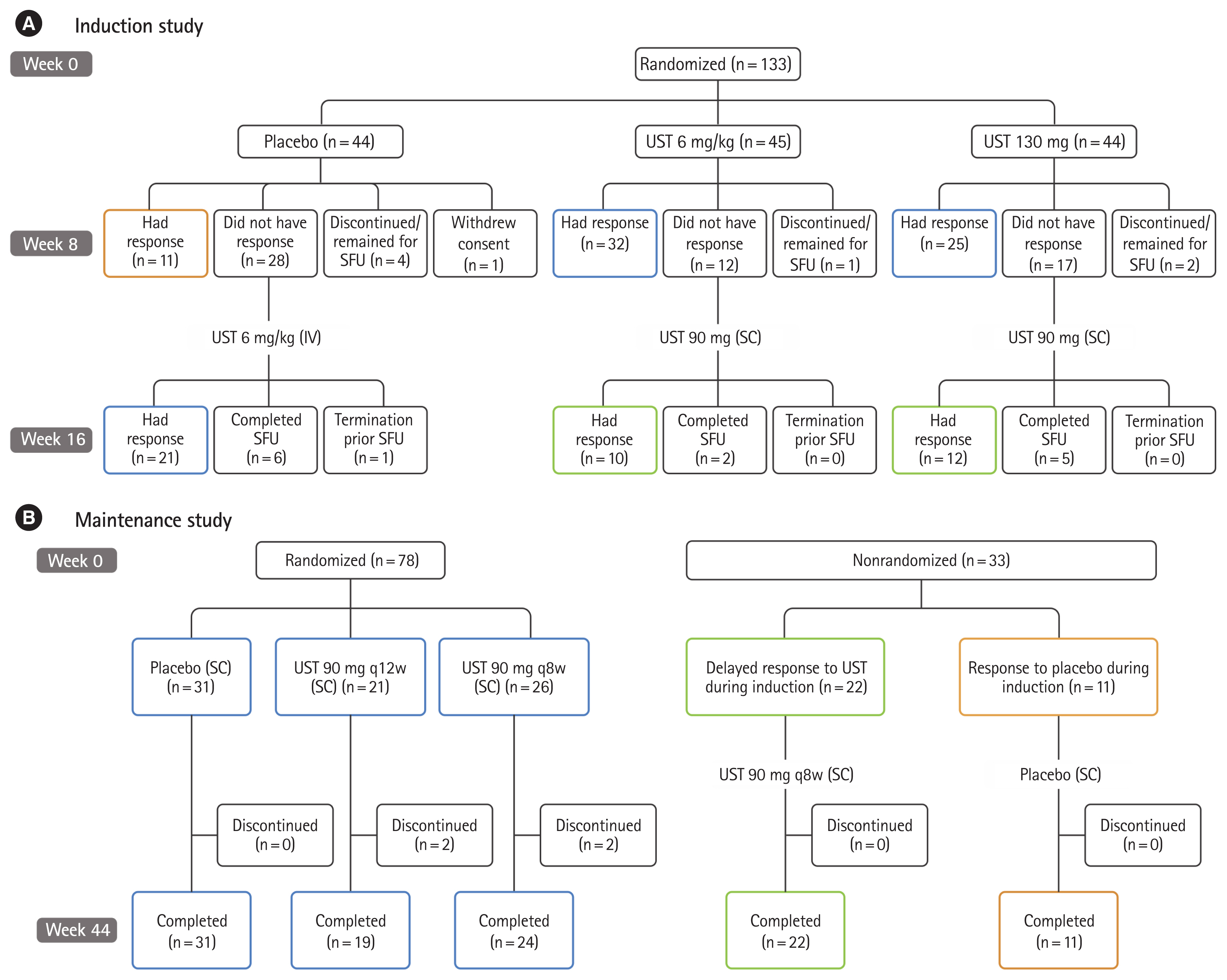
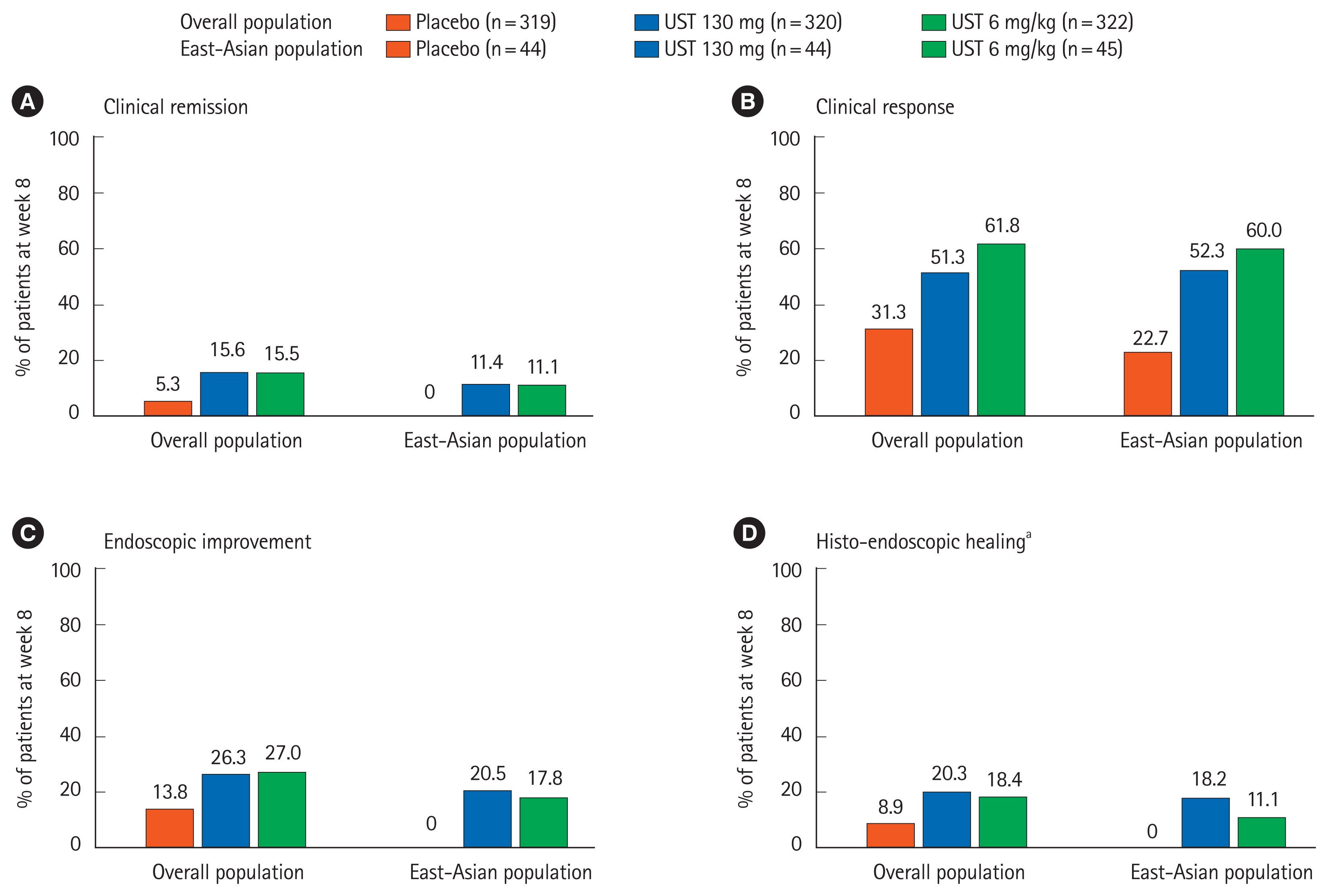
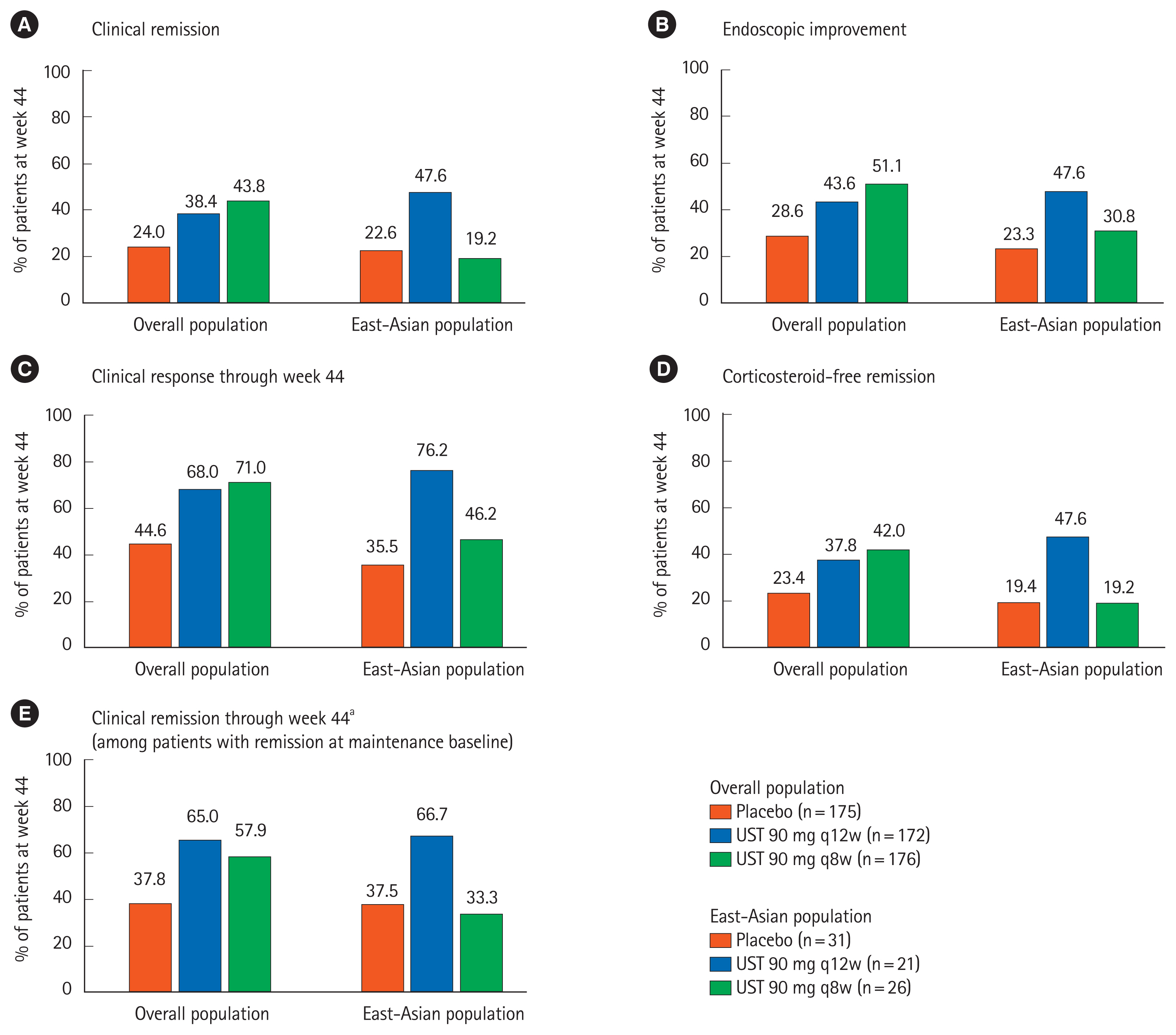
Of 133 East-Asian patients (Japanese: 107, Korean: 26) who underwent randomization, 131 completed induction study and 111 entered maintenance study. In the maintenance study, 78 patients were randomized. Patients who received UST 130 mg and UST 6 mg/kg showed numerically higher clinical remission at week 8 in the induction study (5/44 [11.4%] and 5/45 [11.1%], respectively) compared with those who received placebo (0/44, 0%). The proportion of patients achieved clinical remission at week 44 was numerically higher in the UST 90 mg q12w group (10/21, 47.6%), but similar in the UST 90 mg q8w group (5/26, 19.2%) compared to placebo (7/31, 22.6%). Serious adverse events were reported in 1 patient in UST 130 mg group, but no patient in UST 6 mg/kg group through week 8 in the induction study, and 1 patient in UST 90 mg q12w group and 5 patients in the UST 90 mg q8w group in the maintenance study. No deaths were reported in East-Asian patients throughout the study.
Conclusions
UST induction and maintenance treatments were effective in East-Asian patients with moderate to severe UC; the efficacy and safety profiles were consistent with the overall population.
Keyword
Figure
Cited by 5 articles
-
The Risk of Tuberculosis in Patients With Inflammatory Bowel Disease Treated With Vedolizumab or Ustekinumab in Korea
Myeong Geun Choi, Byong Duk Ye, Suk-Kyun Yang, Tae Sun Shim, Kyung-Wook Jo, Sang Hyoung Park
J Korean Med Sci. 2022;37(14):e107. doi: 10.3346/jkms.2022.37.e107.Natural history of inflammatory bowel disease: a comparison between the East and the West
Eun Mi Song, Suk-Kyun Yang
Intest Res. 2022;20(4):418-430. doi: 10.5217/ir.2021.00104.Concomitant ankylosing spondylitis can increase the risk of biologics or small molecule therapies to control inflammatory bowel disease
Yu Kyung Jun, Hyuk Yoon, Seong-Joon Koh, A Hyeon Kim, Kwang Woo Kim, Jun Won Park, Hyun Jung Lee, Hyoun Woo Kang, Jong Pil Im, Young Soo Park, Joo Sung Kim
Intest Res. 2023;21(2):244-251. doi: 10.5217/ir.2022.00057.Reviewing not Homer’s
Iliad , but “Kai Bao Ben Cao ”: indigo dye—the past, present, and future
Yusuke Yoshimatsu, Tomohisa Sujino, Takanori Kanai
Intest Res. 2023;21(2):174-176. doi: 10.5217/ir.2022.00018.Risks of colorectal cancer and biliary cancer according to accompanied primary sclerosing cholangitis in Korean patients with ulcerative colitis: a nationwide population-based study
Eun Hye Oh, Ye-Jee Kim, Minju Kim, Seung Ha Park, Tae Oh Kim, Sang Hyoung Park
Intest Res. 2023;21(2):252-265. doi: 10.5217/ir.2022.00092.
Reference
-
1. Stenson WF. Inflammatory bowel disease. Goldman l, Bennett JC, editors. Cecil textbook of medicine. 21st ed. Philadelphia: WB Saunders Co;2000. p. 722–729.2. Ng SC. Emerging trends of inflammatory bowel disease in Asia. Gastroenterol Hepatol (N Y). 2016; 12:193–196.3. Murakami Y, Nishiwaki Y, Oba MS, et al. Estimated prevalence of ulcerative colitis and Crohn’s disease in Japan in 2014: an analysis of a nationwide survey. J Gastroenterol. 2019; 54:1070–1077.4. World Gastroenterology Organisation (WGO). WGO handbook on inflammatory bowel disease (IBD): navigating evolving therapies in an evolving disease. Milwaukee: WGO;2017.5. Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016; 14:111–119.6. Kim HJ, Hann HJ, Hong SN, et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006–2012: a nationwide population-based study. Inflamm Bowel Dis. 2015; 21:623–630.7. Choi CH, Jung SA, Lee BI, et al. Diagnostic guideline of ulcerative colitis. Korean J Gastroenterol. 2009; 53:145–160.8. Kornbluth A, Sachar DB. Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010; 105:501–523.
Article9. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017; 11:649–670.
Article10. Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018; 53:305–353.
Article11. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019; 114:384–413.
Article12. Suzuki Y, Motoya S, Hanai H, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol. 2014; 49:283–294.13. Motoya S, Watanabe K, Ogata H, et al. Vedolizumab in Japanese patients with ulcerative colitis: a phase 3, randomized, double-blind, placebo-controlled study. PLoS One. 2019; 14:e0212989.
Article14. Motoya S, Watanabe M, Kim HJ, et al. Tofacitinib induction and maintenance therapy in East Asian patients with active ulcerative colitis: subgroup analyses from three phase 3 multinational studies. Intest Res. 2018; 16:233–245.
Article15. D’Haens GRAM, Lindsay JO, Panaccione R, Schreiber S. Ulcerative colitis: shifting sands. Drugs R D. 2019; 19:227–234.
Article16. Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol. 2011; 106:674–684.
Article17. Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009; 104:760–767.
Article18. Roblin X, Marotte H, Leclerc M, et al. Combination of C-reactive protein, infliximab trough levels, and stable but not transient antibodies to infliximab are associated with loss of response to infliximab in inflammatory bowel disease. J Crohns Colitis. 2015; 9:525–531.
Article19. Vincent FB, Morand EF, Murphy K, Mackay F, Mariette X, Marcelli C. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis. 2013; 72:165–178.
Article20. Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012; 380:1606–1619.
Article21. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017; 376:1723–1736.
Article22. Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014; 147:618–627.
Article23. Bonovas S, Fiorino G, Allocca M, et al. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol. 2016; 14:1385–1397.
Article24. Konidari A, Matary WE. Use of thiopurines in inflammatory bowel disease: safety issues. World J Gastrointest Pharmacol Ther. 2014; 5:63–76.
Article25. Rutgeerts PJ. Review article: the limitations of corticosteroid therapy in Crohn’s disease. Aliment Pharmacol Ther. 2001; 15:1515–1525.26. Lichtenstein GR, Rutgeerts P, Sandborn WJ, et al. A pooled analysis of infections, malignancy, and mortality in infliximab- and immunomodulator-treated adult patients with inflammatory bowel disease. Am J Gastroenterol. 2012; 107:1051–1063.
Article27. Hibi T, Imai Y, Murata Y, Matsushima N, Zheng R, Gasink C. Efficacy and safety of ustekinumab in Japanese patients with moderately to severely active Crohn’s disease: a subpopulation analysis of phase 3 induction and maintenance studies. Intest Res. 2017; 15:475–486.
Article28. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016; 375:1946–1960.29. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019; 381:1201–1214.
Article30. D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007; 132:763–786.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and safety of ustekinumab in Japanese patients with moderately to severely active Crohn's disease: a subpopulation analysis of phase 3 induction and maintenance studies
- Efficacy and safety of mirikizumab as induction and maintenance therapy for Japanese patients with moderately to severely active ulcerative colitis: a subgroup analysis of the global phase 3 LUCENT-1 and LUCENT-2 studies
- Tofacitinib induction and maintenance therapy in East Asian patients with active ulcerative colitis: subgroup analyses from three phase 3 multinational studies
- Efficacy and safety of vedolizumab in ulcerative colitis in patients from Asian countries in the GEMINI 1 study
- Efficacy and Safety of Adalimumab in Moderately to Severely Active Cases of Ulcerative Colitis: A Meta-Analysis of Published Placebo-Controlled Trials