Intest Res.
2024 Jul;22(3):369-377. 10.5217/ir.2023.00194.
Long-term efficacy and safety of tofacitinib in patients with ulcerative colitis: 3-year results from a real-world study
- Affiliations
-
- 1Department of Gastroenterology and Hepatology, Tokyo Medical and Dental University, Tokyo, Japan
- 2First Department of Internal Medicine, Faculty of Medicine, University of Yamanashi, Chuo, Japan
- KMID: 2558196
- DOI: http://doi.org/10.5217/ir.2023.00194
Abstract
- Background/Aims
The efficacy and safety of tofacitinib for the treatment of refractory ulcerative colitis (UC) has been demonstrated in clinical trials. Although, a series of reports with real-world evidence of its short-term efficacy and safety profiles have already been published, reports of long-term real-world data have been limited. We aimed to show our 3-year evidence on the clinical use of tofacitinib for the treatment of UC, focusing on its efficacy and safety profiles.
Methods
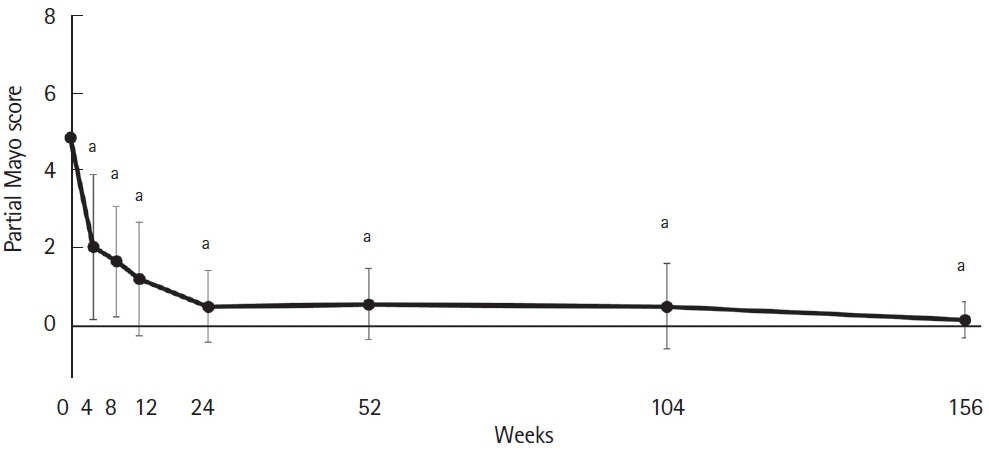
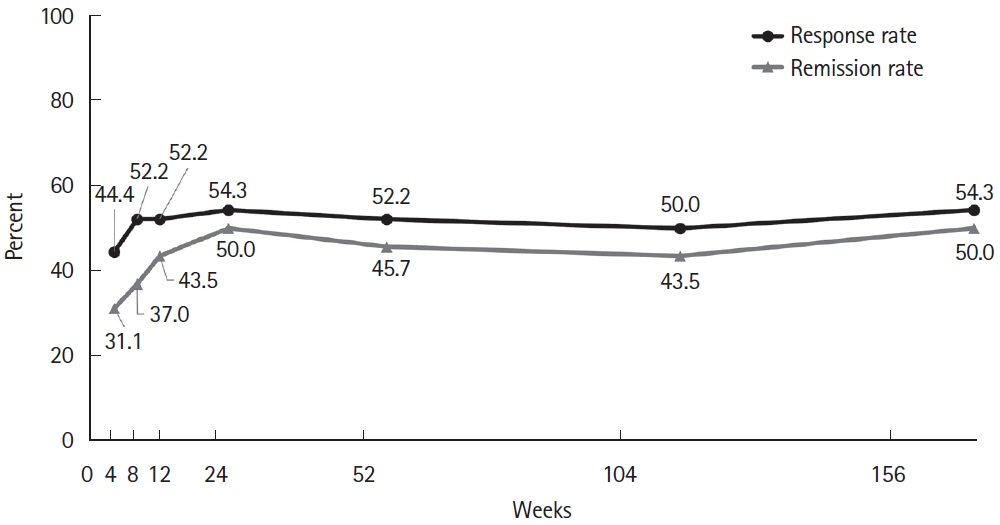
A retrospective observational study was conducted on patients who started tofacitinib for active refractory UC at our hospital. The primary outcome was the retention rate until 156 weeks after initiating tofacitinib. The secondary outcomes were short-term efficacy at 4, 8, and 12 weeks; long-term efficacy at 52, 104, and 156 weeks; prognostic factors related to the cumulative retention rate; loss of response; and safety profile, including adverse events.
Results
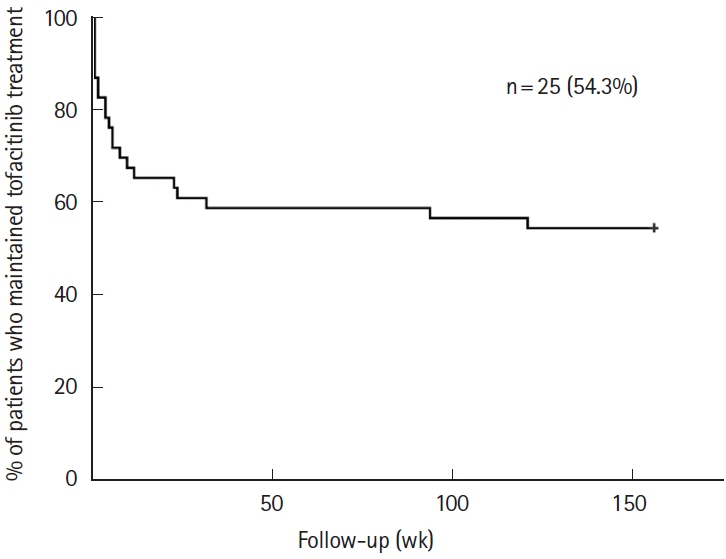
Forty-six patients who were able to be monitored for up to 156 weeks after tofacitinib initiation, were enrolled in this study. Continuation of tofacitinib was possible until 156 weeks in 54.3%, with > 50% response rates and > 40% remission rates. Among patients in whom response or remission was achieved and tofacitinib was deescalated after 8 weeks of induction treatment, 54.3% experienced relapse but were successfully rescued by and retained on reinduction treatment, except for 1 patient. No serious AEs were observed in the study.
Conclusions
Tofacitinib is effective and safe as long-term treatment in a refractory cohort of UC patients in real-world clinical practice.
Keyword
Figure
Reference
-
1. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017; 389:1756–1770.
Article2. Danese S, Grisham M, Hodge J, Telliez JB. JAK inhibition using tofacitinib for inflammatory bowel disease treatment: a hub for multiple inflammatory cytokines. Am J Physiol Gastrointest Liver Physiol. 2016; 310:G155–G162.
Article3. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017; 376:1723–1736.
Article4. Sands BE, Armuzzi A, Marshall JK, et al. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: results from OCTAVE Open. Aliment Pharmacol Ther. 2020; 51:271–280.
Article5. Colombel JF, Osterman MT, Thorpe AJ, et al. Maintenance of remission with tofacitinib therapy in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2022; 20:116–125.
Article6. Sandborn WJ, Panés J, D’Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol. 2019; 17:1541–1550.7. Vermeire S, Su C, Lawendy N, et al. Outcomes of tofacitinib dose reduction in patients with ulcerative colitis in stable remission from the randomised RIVETING trial. J Crohns Colitis. 2021; 15:1130–1141.
Article8. Shimizu H, Fujii T, Hibiya S, et al. Rapid prediction of 1-year efficacy of tofacitinib for treating refractory ulcerative colitis. Intest Res. 2021; 19:115–118.
Article9. Taxonera C, Olivares D, Alba C. Real-world effectiveness and safety of tofacitinib in patients with ulcerative colitis: systematic review with meta-analysis. Inflamm Bowel Dis. 2022; 28:32–40.
Article10. Biemans VB, Sleutjes JA, de Vries AC, et al. Tofacitinib for ulcerative colitis: results of the prospective Dutch Initiative on Crohn and Colitis (ICC) registry. Aliment Pharmacol Ther. 2020; 51:880–888.
Article11. Honap S, Chee D, Chapman TP, et al. Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multicentre UK experience. J Crohns Colitis. 2020; 14:1385–1393.12. Chaparro M, Garre A, Mesonero F, et al. Tofacitinib in ulcerative colitis: real-world evidence from the ENEIDA registry. J Crohns Colitis. 2021; 15:35–42.
Article13. Ishida N, Miyazu T, Tamura S, et al. Real-world efficacy and safety monitoring for predicting continuation of tofacitinib therapy in patients with ulcerative colitis. Dig Dis Sci. 2022; 67:3984–3992.
Article14. Shin SH, Oh K, Hong SN, et al. Real-life effectiveness and safety of tofacitinib treatment in patients with ulcerative colitis: a KASID multicenter cohort study. Therap Adv Gastroenterol. 2023; 16:17562848231154103.
Article15. Straatmijer T, van Schaik FD, Bodelier AG, et al. Effectiveness and safety of tofacitinib for ulcerative colitis: two-year results of the ICC Registry. Aliment Pharmacol Ther. 2023; 57:117–126.
Article16. Yu A, Ha NB, Shi B, Cheng YW, Mahadevan U, Beck KR. Realworld experience with tofacitinib dose de-escalation in patients with moderate and severe ulcerative colitis. Clin Gastroenterol Hepatol. 2023; 21:3115–3124.
Article17. Long MD, Afzali A, Fischer M, et al. Tofacitinib Response in Ulcerative Colitis (TOUR): early response after initiation of tofacitinib therapy in a real-world setting. Inflamm Bowel Dis. 2023; 29:570–578.
Article18. Mukherjee A, Hazra A, Smith MK, et al. Exposure-response characterization of tofacitinib efficacy in moderate to severe ulcerative colitis: results from a dose-ranging phase 2 trial. Br J Clin Pharmacol. 2018; 84:1136–1145.
Article19. Irving PM, Leung Y, Dubinsky MC. Review article: guide to tofacitinib dosing in patients with ulcerative colitis. Aliment Pharmacol Ther. 2022; 56:1131–1145.
Article20. Deepak P, Alayo QA, Khatiwada A, et al. Safety of tofacitinib in a real-world cohort of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2021; 19:1592–1601.
Article21. U.S. Food and Drug Administration (FDA). Food and Drug Administration (FDA). FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions [Internet]. c2021. [cited 2023 Jul 6]. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death.22. Sandborn WJ, Peyrin-Biroulet L, Quirk D, et al. Efficacy and safety of extended induction with tofacitinib for the treatment of ulcerative colitis. Clin Gastroenterol Hepatol. 2022; 20:1821–1830.
Article23. Winthrop KL, Vermeire S, Long MD, et al. Long-term risk of herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis. 2023; 29:85–96.
Article24. Winthrop KL, Melmed GY, Vermeire S, et al. Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis. 2018; 24:2258–2265.
Article25. Din S, Selinger CP, Black CJ, Ford AC. Systematic review with network meta-analysis: risk of Herpes zoster with biological therapies and small molecules in inflammatory bowel disease. Aliment Pharmacol Ther. 2023; 57:666–675.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of inflammatory bowel diseases: focusing on biologic agents and new therapies
- Effects of Dextran Sulfate Sodium-Induced Ulcerative Colitis on the Disposition of Tofacitinib in Rats
- The Comparative Risk of Serious Adverse Events With Tofacitinib and TNF Inhibitors in Patients With Ulcerative Colitis: The Korean Experience as Revealed by a National Database
- The Efficacy and Safety of a Tofacitinib in the Treatment of Active Ulcerative Colitis
- Advancements in the Management of Moderate-to-Severe Ulcerative Colitis: A Revised 2023 Korean Treatment Guidelines