J Vet Sci.
2018 Jul;19(4):500-504. 10.4142/jvs.2018.19.4.500.
Oxidative stress and hepatic injury induced in mice fed a Sarcocystis hirsuta cyst extract
- Affiliations
-
- 1Department of Pathobiology, Faculty of Veterinary Medicine, University of Zabol, Zabol 98613635856, Iran. nabavir796@gmail.com
- 2Department of Basic Veterinary Science, Faculty of Veterinary Medicine, University of Zabol, Zabol 98613635856, Iran.
- 3Department of Biology, School of Basic Sciences, University of Zabol, Zabol 98613635856, Iran.
- KMID: 2417564
- DOI: http://doi.org/10.4142/jvs.2018.19.4.500
Abstract
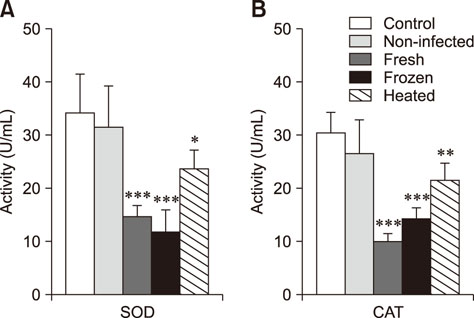
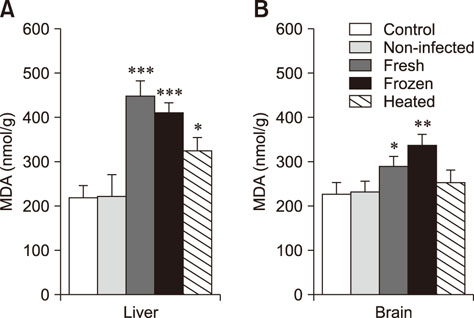
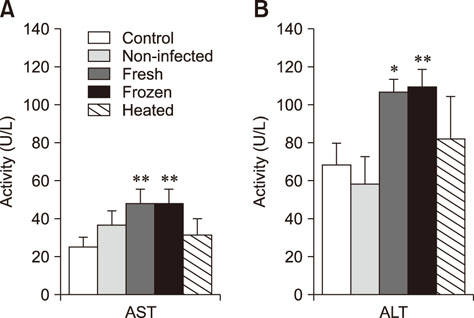
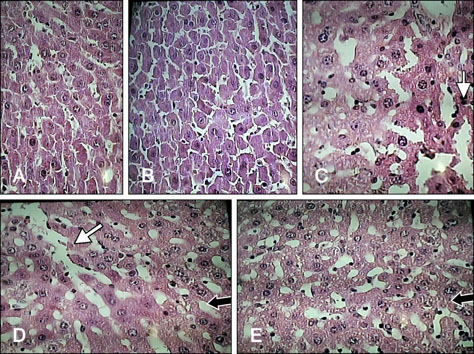
- We studied the toxic effects of a Sarcocystis hirsuta cyst extract fed to mice. Degenerative changes were found in mice gavage-fed fresh, frozen, and heat-treated S. hirsuta cyst extract. There were increases in the levels of serum aspartate aminotransferase and alanine aminotransferase as well as hepatic and brain malondialdehyde (MDA) levels along with concomitant decreases in catalase (CAT) and superoxide dismutase (SOD) activities of mice receiving fresh and frozen S. hirsuta extracts. Gavage feeding of heat-treated S. hirsuta cyst extract had no effects on liver enzymes or brain MDA content, but the liver MDA level did increase. Mice in the heat-treated cyst group showed reduced CAT and SOD activities as well as increased hepatic MDA levels compared to those in the control group. These results indicate that an extract of S. hirsuta cyst can induce oxidative stress and hepatic injury, even after heat treatment.
Keyword
MeSH Terms
Figure
Reference
-
1. Al-Hyali NS, Khalil LY, Aljawady MA. Sarcotoxin effect on leukocytic finding and phagocytic activity in mice. J Anim Vet Adv. 2009; 8:2395–2398.2. Bunyaratvej S, Bunyawongwiroj P, Nitiyanant P. Human intestinal sarcosporidiosis: report of six cases. Am J Trop Med Hyg. 1982; 31:36–41.3. Drotman RB, Lawhorn GT. Serum enzymes as indicators of chemically induced liver damage. Drug Chem Toxicol. 1978; 1:163–171.
Article4. el-Akkad IN, Mandour AM. On some pharmacological and toxicological effects of a protozoan toxin “sarcocystin”. J Egypt Med Assoc. 1969; 52:942–948.5. Fayer R, Esposito DH, Dubey JP. Human infections with Sarcocystis species. Clin Microbiol Rev. 2015; 28:295–311.6. Gjerde B. Molecular characterisation of Sarcocystis bovifelis, Sarcocystis bovini n. sp., Sarcocystis hirsuta and Sarcocystis cruzi from cattle (Bos taurus) and Sarcocystis sinensis from water buffaloes (Bubalus bubalis). Parasitol Res. 2016; 115:1473–1492.
Article7. Hosseini H, Khaksar R, Shemshadi B. Study on infestation of raw hamburgers to Sarcocystis cyst in Tehran. Iranian Nut Sci. 2007; 4:65–70.8. Jahed-Khaniki GR, Kia EB. Detection of Sarcocystis cysts from meat supplied for hamburger in Iran by histological method. J Med Sci. 2006; 6:18–21.9. Lau YL, Chang PY, Tan CT, Fong MY, Mahmud R, Wong KT. Sarcocystis nesbitti infection in human skeletal muscle: possible transmission from snakes. Am J Trop Med Hyg. 2014; 90:361–364.
Article10. Lei XG, Zhu JH, Cheng WH, Bao Y, Ho YS, Reddi AR, Holmgren A, Arnér ES. Paradoxical roles of antioxidant enzymes: basic mechanisms and health implications. Physiol Rev. 2016; 96:307–364.
Article11. Lhafi KS, Mitzscherling TA, Kühne M. [Parasites in meat: a challenge for veterinarians in meat hygiene]. Dtsch Tierarztl Wochenschr. 2004; 111:277–281. German.12. Lindsay DS, Blagburn BL, Braund KG. Sarcocystis spp. and sarcocystosis. Br Med J. 1995; 5:249–254.13. Lunde MN, Jacobs L. Properties of toxoplasma lysates toxic to rabbits on intravenous injection. J Parasitol. 1964; 50:49–51.
Article14. Mandour AM. Studies on the toxicity of Sarcocystis. J Med Microbiol. 1969; 2:361–363.15. Mirzaei M, Rezaei H. A survey on Sarcocystis spp. infection in cattle of Tabriz city, Iran. J Parasit Dis. 2016; 40:648–651.
Article16. Najafiyan HR, Mohebali M, Keshavarz H. Study on frequency of Sarcocystis spp. by macroscopic and microscopic methods in slaughtered cattle in Shahriar district and their public health importance. Paj Saz. 2007; 77:15–19.17. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358.
Article18. Saleque A, Bhatia BB, Juyal PD, Rahman H. Toxicity of cyst extract of Sarcocystis fusiformis from buffalo in rabbits and mice. Vet Parasitol. 1991; 38:61–65.
Article19. Shekarforoush SS, Razavi SM, Abbasvali M. First detection of Sarcocystis hirsuta from cattle in Iran. Iran J Vet Res. 2013; 14:155–157.20. Sim MK. Cardiovascular actions of chicken-meat extract in normo- and hypertensive rats. Br J Nutr. 2001; 86:97–103.
Article21. Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol. 2001; 280:C53–C60.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gamma-tocopherol ameliorates hyperglycemia-induced hepatic inflammation associated with NLRP3 inflammasome in alloxan-induced diabetic mice
- Green perilla leaf extract ameliorates long-term oxidative stress induced by a high-fat diet in aging mice
- Hepatic ischemia-reperfusion injury with respect to oxidative stress and inflammatory response: a narrative review
- Folic acid supplementation reduces oxidative stress and hepatic toxicity in rats treated chronically with ethanol
- Carbon monoxide releasing molecule-2 protects mice against acute kidney injury through inhibition of ER stress