Ann Dermatol.
2018 Apr;30(2):143-149. 10.5021/ad.2018.30.2.143.
Risk Factors Affecting Adverse Effects of Cyclosporine A in a Real-World Psoriasis Treatment
- Affiliations
-
- 1Department of Dermatology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. swyoun@snu.ac.kr
- KMID: 2414674
- DOI: http://doi.org/10.5021/ad.2018.30.2.143
Abstract
- BACKGROUND
No study to date has focused on the changes in laboratory test results and related risk factors in patients with psoriasis treated with prolonged Cyclosporine A (CsA) therapy.
OBJECTIVE
The objective of this study was to investigate the changes of laboratory values and related risk factors in patients with psoriasis treated with CsA in a real-world setting.
METHODS
Records of patients with psoriasis treated with CsA at an outpatient clinic were collected, and a Cox proportional hazards regression model was used.
RESULTS
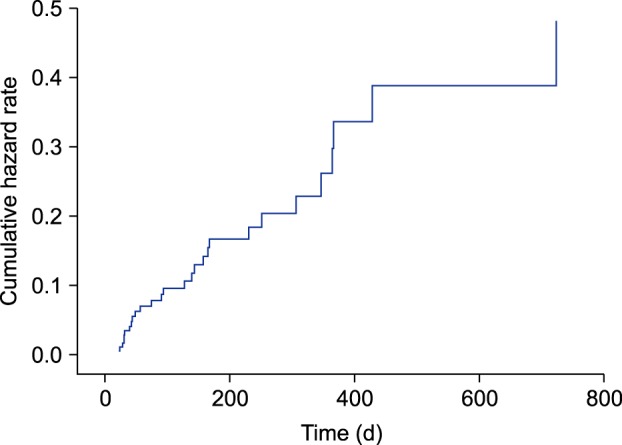
Of the 128 patients included in this study, 28 patients (21.9%) showed laboratory test abnormalities over a mean medication period of 11.6 months. Older age (hazard ratio [HR], 1.174; 95% confidence interval [CI], 1.068~1.370; p=0.007) and pre-existing kidney disease (HR, 0.008; 95% CI, 0~0.205; p=0.001) significantly increased the risk of renal dysfunction. Male sex was the only significant risk factor for liver enzyme elevation (HR, 0.284; 95% CI, 0.081~0.784; p=0.026) and uric acid abnormality (HR, 0.048; 95% CI, 0~0.372; p=0.046).
CONCLUSION
This is an in-depth analysis of laboratory changes and related risk factors in patients with psoriasis treated with CsA. Liver is the most commonly affected organ of CsA toxicity. Older age, male sex, and presence of kidney disease were risk factors associated with laboratory abnormality during CsA treatment.
MeSH Terms
Figure
Cited by 1 articles
-
Recent medical therapy for psoriasis
Sang Woong Youn
J Korean Med Assoc. 2019;62(3):176-180. doi: 10.5124/jkma.2019.62.3.176.
Reference
-
1. Griffiths CE, Clark CM, Chalmers RJ, Li Wan Po A, Williams HC. A systematic review of treatments for severe psoriasis. Health Technol Assess. 2000; 4:1–125.
Article2. Amor KT, Ryan C, Menter A. The use of cyclosporine in dermatology: part I. J Am Acad Dermatol. 2010; 63:925–946. PMID: 21093659.
Article3. Markham T, Watson A, Rogers S. Adverse effects with long-term cyclosporin for severe psoriasis. Clin Exp Dermatol. 2002; 27:111–114. PMID: 11952700.
Article4. García-Bustínduy M, Escoda M, Guimerá FJ, Sáez M, Dorta S, Fagundo E, et al. Safety of long-term treatment with cyclosporin A in resistant chronic plaque psoriasis: a retrospective case series. J Eur Acad Dermatol Venereol. 2004; 18:169–172. PMID: 15009296.5. Lebwohl M, Ellis C, Gottlieb A, Koo J, Krueger G, Linden K, et al. Cyclosporine consensus conference: with emphasis on the treatment of psoriasis. J Am Acad Dermatol. 1998; 39:464–475. PMID: 9738783.
Article6. Grossman RM, Chevret S, Abi-Rached J, Blanchet F, Dubertret L. Long-term safety of cyclosporine in the treatment of psoriasis. Arch Dermatol. 1996; 132:623–629. PMID: 8651712.
Article7. Rosmarin DM, Lebwohl M, Elewski BE, Gottlieb AB. National Psoriasis Foundation. Cyclosporine and psoriasis: 2008 National Psoriasis Foundation Consensus Conference. J Am Acad Dermatol. 2010; 62:838–853. PMID: 19932926.
Article8. Griffiths CE, Dubertret L, Ellis CN, Finlay AY, Finzi AF, Ho VC, et al. Ciclosporin in psoriasis clinical practice: an international consensus statement. Br J Dermatol. 2004; 150(Suppl 67):11–23.
Article9. Berth-Jones J. The use of ciclosporin in psoriasis. J Dermatolog Treat. 2005; 16:258–277. PMID: 16428145.10. Colombo MD, Cassano N, Bellia G, Vena GA. Cyclosporine regimens in plaque psoriasis: an overview with special emphasis on dose, duration, and old and new treatment approaches. ScientificWorldJournal. 2013; 2013:805705. PMID: 23983647.
Article11. Yoon HS, Youn JI. A comparison of two cyclosporine dosage regimens for the treatment of severe psoriasis. J Dermatolog Treat. 2007; 18:286–290. PMID: 17852632.
Article12. Kim SY, Yun SJ, Lee JB, Kim SJ, Won YH, Lee SC. A comparison of efficacy and adverse reactions between methotrexate and cyclosporine A. Korean J Dermatol. 2014; 52:615–621.13. Kim MJ, Kim HS, Kim HO, Park YM. The efficacy, safety and long-term effect of cyclosporine for treating chronic idiopathic urticaria. Korean J Dermatol. 2009; 47:759–764.14. Haw S, Shin MK, Haw CR. The efficacy and safety of long-term oral cyclosporine treatment for patients with atopic dermatitis. Ann Dermatol. 2010; 22:9–15. PMID: 20548874.
Article15. Park HC, Kim E, Kim JE, Ko JY, Ro YS. Adverse effects of oral cyclosporine in the treatment of skin diseases. Korean J Dermatol. 2013; 51:960–969.16. Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009; 61:451–485. PMID: 19493586.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Retrospective Analysis of the Factors Affecting the Treatment Outcomes of Cyclosporine in Patients with Psoriasis
- Real-World Experience with Etanercept Therapy and the Switching Pattern among Korean Patients with Psoriasis after Withdrawal of Etanercept
- A Case of a Patient with Psoriasis Aggravated by Scabies Infstation
- Infliximab: Effective Therapy for Pustular Psoriasis
- A Case of Rebound Phenomenon after Cessation of Cyclosporine for Guselkumab Therapy in Psoriasis