Korean J Physiol Pharmacol.
2018 Jul;22(4):447-456. 10.4196/kjpp.2018.22.4.447.
Angiotensin-(1-9) ameliorates pulmonary arterial hypertension via angiotensin type II receptor
- Affiliations
-
- 1Department of Physiology, Research Institute for Endocrine Sciences, Chonbuk National University Medical School, Jeonju 54907, Korea. shkim@chonbuk.ac.kr
- KMID: 2414269
- DOI: http://doi.org/10.4196/kjpp.2018.22.4.447
Abstract
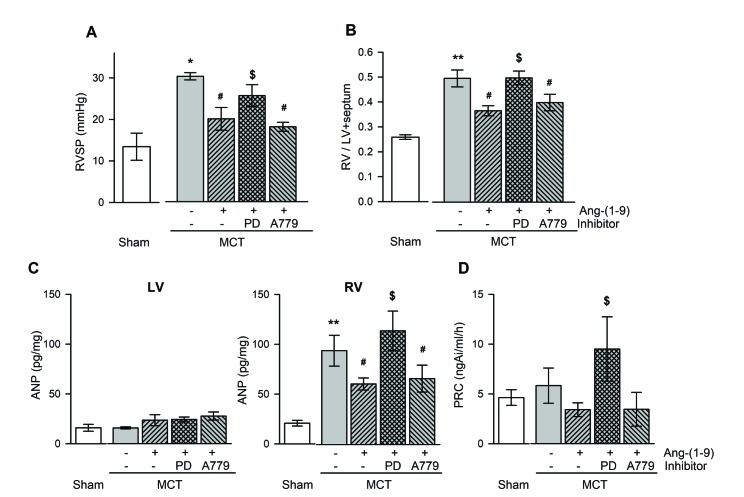
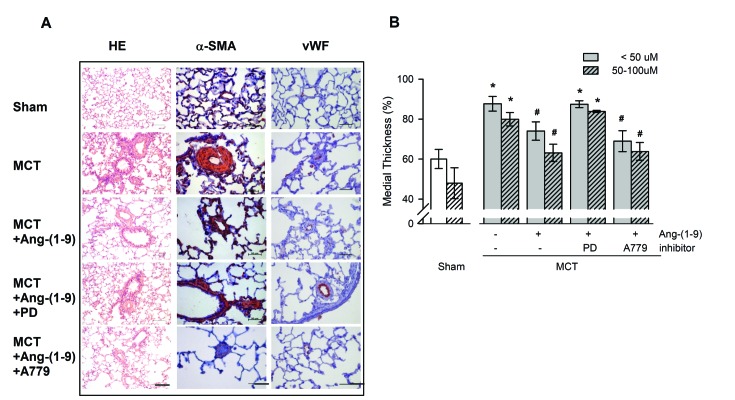
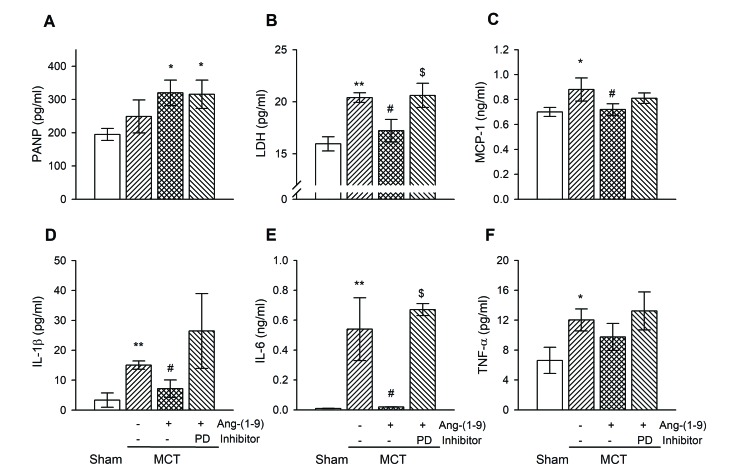
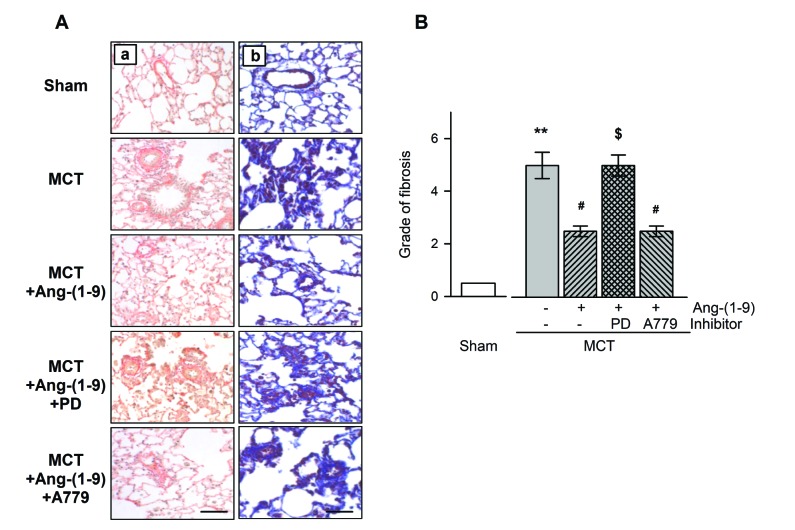
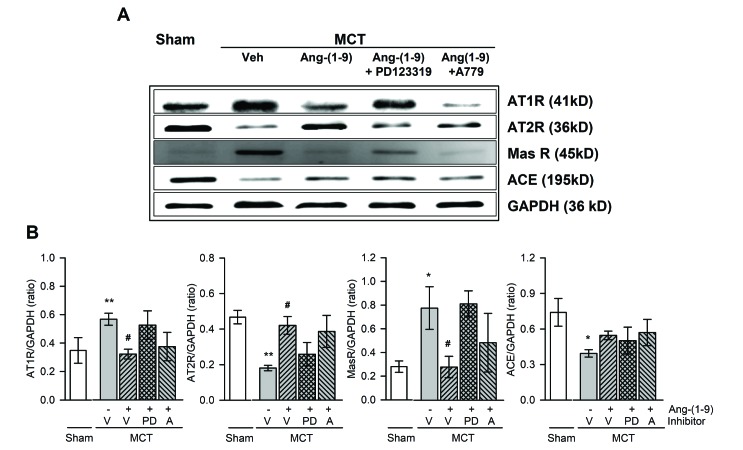
- Angiotensin-(1-9) [Ang-(1-9)], generated from Ang I by Ang II converting enzyme 2, has been reported to have protective effects on cardiac and vascular remodeling. However, there is no report about the effect of Ang-(1-9) on pulmonary hypertension. The aim of the present study is to investigate whether Ang-(1-9) improves pulmonary vascular remodeling in monocrotaline (MCT)-induced pulmonary hypertensive rats. Sprague-Dawley rats received Ang-(1-9) (576 µg/kg/day) or saline via osmotic mini-pumps for 3 weeks. Three days after implantation of osmotic mini-pumps, 50 mg/kg MCT or vehicle were subcutaneously injected. MCT caused increases in right ventricular weight and systolic pressure, which were reduced by co-administration of Ang-(1-9). Ang-(1-9) also attenuated endothelial damage and medial hypertrophy of pulmonary arterioles as well as pulmonary fibrosis induced by MCT. The protective effects of Ang-(1-9) against pulmonary hypertension were inhibited by Ang type 2 receptor (ATâ‚‚R) blocker, but not by Mas receptor blocker. Additionally, the levels of LDH and inflammatory cytokines, such as TNF-α, MCP-1, IL-1β, and IL-6, in plasma were lower in Ang-(1-9) co-treated MCT group than in vehicle-treated MCT group. Changes in expressions of apoptosis-related proteins such as Bax, Bcl-2, Caspase-3 and -9 in the lung tissue of MCT rats were attenuated by the treatment with Ang-(1-9). These results indicate that Ang-(1-9) improves MCT-induced pulmonary hypertension by decreasing apoptosis and inflammatory reaction via ATâ‚‚R.
MeSH Terms
-
Angiotensins*
Animals
Apoptosis
Arterioles
Blood Pressure
Caspase 3
Cytokines
Hypertension*
Hypertension, Pulmonary
Hypertrophy
Interleukin-6
Lung
Monocrotaline
Plasma
Pulmonary Fibrosis
Rats
Rats, Sprague-Dawley
Receptor, Angiotensin, Type 2
Vascular Remodeling
Angiotensins
Caspase 3
Cytokines
Interleukin-6
Monocrotaline
Receptor, Angiotensin, Type 2
Figure
Reference
-
1. Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2008; 118:2372–2379. PMID: 18596905.
Article2. Marshall RP. The pulmonary renin-angiotensin system. Curr Pharm Des. 2003; 9:715–722. PMID: 12570789.
Article3. Flores-Munoz M, Work LM, Douglas K, Denby L, Dominiczak AF, Graham D, Nicklin SA. Angiotensin-(1-9) attenuates cardiac fibrosis in the stroke-prone spontaneously hypertensive rat via the angiotensin type 2 receptor. Hypertension. 2012; 59:300–307. PMID: 22184331.
Article4. Ocaranza MP, Moya J, Barrientos V, Alzamora R, Hevia D, Morales C, Pinto M, Escudero N, García L, Novoa U, Ayala P, Díaz-Araya G, Godoy I, Chiong M, Lavandero S, Jalil JE, Michea L. Angiotensin-(1-9) reverses experimental hypertension and cardiovascular damage by inhibition of the angiotensin converting enzyme/Ang II axis. J Hypertens. 2014; 32:771–783. PMID: 24463937.
Article5. Ocaranza MP, Rivera P, Novoa U, Pinto M, González L, Chiong M, Lavandero S, Jalil JE. Rho kinase inhibition activates the homologous angiotensin-converting enzyme-angiotensin-(1-9) axis in experimental hypertension. J Hypertens. 2011; 29:706–715. PMID: 21330937.
Article6. Jackman HL, Massad MG, Sekosan M, Tan F, Brovkovych V, Marcic BM, Erdös EG. Angiotensin 1-9 and 1-7 release in human heart: role of cathepsin A. Hypertension. 2002; 39:976–981. PMID: 12019279.7. Bruce E, Shenoy V, Rathinasabapathy A, Espejo A, Horowitz A, Oswalt A, Francis J, Nair A, Unger T, Raizada MK, Steckelings UM, Sumners C, Katovich MJ. Selective activation of angiotensin AT2 receptors attenuates progression of pulmonary hypertension and inhibits cardiopulmonary fibrosis. Br J Pharmacol. 2015; 172:2219–2231. PMID: 25522140.8. Cha SA, Park BM, Gao S, Kim SH. Stimulation of ANP by angiotensin-(1-9) via the angiotensin type 2 receptor. Life Sci. 2013; 93:934–940. PMID: 24177599.
Article9. Flores-Muñoz M, Smith NJ, Haggerty C, Milligan G, Nicklin SA. Angiotensin1-9 antagonises pro-hypertrophic signalling in cardiomyocytes via the angiotensin type 2 receptor. J Physiol. 2011; 589:939–951. PMID: 21173078.
Article10. Oh YB, Kim JH, Park BM, Park BH, Kim SH. Captopril intake decreases body weight gain via angiotensin-(1-7). Peptides. 2012; 37:79–85. PMID: 22743141.
Article11. Gao S, Oh YB, Shah A, Park WH, Chung MJ, Lee YH, Kim SH. Urotensin II receptor antagonist attenuates monocrotaline-induced cardiac hypertrophy in rats. Am J Physiol Heart Circ Physiol. 2010; 299:H1782–H1789. PMID: 20870804.
Article12. Cho KW, Kim SH, Koh GY, Seul KH, Huh KS, Chu D, Rapp NS, Moon HB, Kim KK, Kook YJ. Plasma concentration of atrial natriuretic peptide in different phases of Korean hemorrhagic fever. Nephron. 1989; 51:215–219. PMID: 2563575.
Article13. Nishii Y, Gabazza EC, Fujimoto H, Nakahara H, Takagi T, Bruno N, D'Alessandro-Gabazza CN, Maruyama J, Maruyama K, Hayashi T, Adachi Y, Suzuki K, Taguchi O. Protective role of protein C inhibitor in monocrotaline-induced pulmonary hypertension. J Thromb Haemost. 2006; 4:2331–2339. PMID: 17059470.
Article14. Schultze AE, Gunaga KP, Wagner JG, Hoorn CM, Moorehead WR, Roth RA. Lactate dehydrogenase activity and isozyme patterns in tissues and bronchoalveolar lavage fluid from rats treated with monocrotaline pyrrole. Toxicol Appl Pharmacol. 1994; 126:301–310. PMID: 8209383.
Article15. Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988; 41:467–470. PMID: 3366935.
Article16. Hübner RH, Gitter W, El Mokhtari NE, Mathiak M, Both M, Bolte H, Freitag-Wolf S, Bewig B. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques. 2008; 44:507–511. 514–517. PMID: 18476815.
Article17. Ikeda Y, Yonemitsu Y, Kataoka C, Kitamoto S, Yamaoka T, Nishida K, Takeshita A, Egashira K, Sueishi K. Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol. 2002; 283:H2021–H2028. PMID: 12384481.
Article18. Ocaranza MP, Godoy I, Jalil JE, Varas M, Collantes P, Pinto M, Roman M, Ramirez C, Copaja M, Diaz-Araya G, Castro P, Lavandero S. Enalapril attenuates downregulation of Angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension. 2006; 48:572–578. PMID: 16908757.
Article19. Ocaranza MP, Lavandero S, Jalil JE, Moya J, Pinto M, Novoa U, Apablaza F, Gonzalez L, Hernandez C, Varas M, Lopez R, Godoy I, Verdejo H, Chiong M. Angiotensin-(1-9) regulates cardiac hypertrophy in vivo and in vitro. J Hypertens. 2010; 28:1054–1064. PMID: 20411619.
Article20. Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, Voelkel NF, Bogaard HJ. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol. 2012; 302:L363–L369. PMID: 21964406.
Article21. Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006; 99:675–691. PMID: 17008597.22. Ocaranza MP, Michea L, Chiong M, Lagos CF, Lavandero S, Jalil JE. Recent insights and therapeutic perspectives of angiotensin-(1-9) in the cardiovascular system. Clin Sci (Lond). 2014; 127:549–557. PMID: 25029123.
Article23. Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012; 186:261–272. PMID: 22679007.
Article24. Cool CD, Kennedy D, Voelkel NF, Tuder RM. Pathogenesis and evolution of plexiform lesions in pulmonary hypertension associated with scleroderma and human immunodeficiency virus infection. Hum Pathol. 1997; 28:434–442. PMID: 9104943.
Article25. Shenoy V, Ferreira AJ, Qi Y, Fraga-Silva RA, Díez-Freire C, Dooies A, Jun JY, Sriramula S, Mariappan N, Pourang D, Venugopal CS, Francis J, Reudelhuber T, Santos RA, Patel JM, Raizada MK, Katovich MJ. The angiotensin-converting enzyme 2/angiogenesis-(1-7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am J Respir Crit Care Med. 2010; 182:1065–1072. PMID: 20581171.
Article26. Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin P, Walker C, Budd DC, Pepke-Zaba J, Morrell NW. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010; 122:920–927. PMID: 20713898.
Article27. Tuder RM, Voelkel NF. Pulmonary hypertension and inflammation. J Lab Clin Med. 1998; 132:16–24. PMID: 9665367.
Article28. Sutendra G, Dromparis P, Bonnet S, Haromy A, McMurtry MS, Bleackley RC, Michelakis ED. Pyruvate dehydrogenase inhibition by the inflammatory cytokine TNFα contributes to the pathogenesis of pulmonary arterial hypertension. J Mol Med (Berl). 2011; 89:771–783. PMID: 21809123.
Article29. Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008; 29:367–374. PMID: 18579222.
Article30. Morrell NW, Upton PD, Kotecha S, Huntley A, Yacoub MH, Polak JM, Wharton J. Angiotensin II activates MAPK and stimulates growth of human pulmonary artery smooth muscle via AT1 receptors. Am J Physiol. 1999; 277:L440–L448. PMID: 10484450.31. de Man FS, Tu L, Handoko ML, Rain S, Ruiter G, François C, Schalij I, Dorfmüller P, Simonneau G, Fadel E, Perros F, Boonstra A, Postmus PE, van der Velden J, Vonk-Noordegraaf A, Humbert M, Eddahibi S, Guignabert C. Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012; 186:780–789. PMID: 22859525.
Article32. Wang Y, Zhang XH, Wang HL. Involvement of BMPR2 in the protective effect of fluoxetine against monocrotaline-induced endothelial apoptosis in rats. Can J Physiol Pharmacol. 2011; 89:345–354. PMID: 21619414.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hyperkalemia from preoperative non-steroidal anti-inflammatory drugs and angiotensin II receptor blockers in patients with nephropathy
- Renin Angiotensin System in Rabbit Corpus Cavernosum: Functional Characterization of Angiotensin II Receptors
- Association Analysis of a Polymorphism of the Angiotensin I-Converting Enzyme Gene and Angiotensin II Type 1 Receptor Gene in Korean Population
- Effects of Angiotensin II on ZO-1 in Glomerular Epithelial Cells
- Lithium Intoxication Induced by Angiotensin II Receptor Blocker/Thiazide Combination Agent