Lab Anim Res.
2018 Jun;34(2):80-83. 10.5625/lar.2018.34.2.80.
Elimination half-lives of interleukin-6 and cytokine-induced neutrophil chemoattractant-1 synthesized in response to inflammatory stimulation in rats
- Affiliations
-
- 1Laboratory of Immunology, School of Life and Environmental Science, Azabu University, Kanagawa, Japan. kuirbaya@azabu-u.ac.jp
- KMID: 2413847
- DOI: http://doi.org/10.5625/lar.2018.34.2.80
Abstract
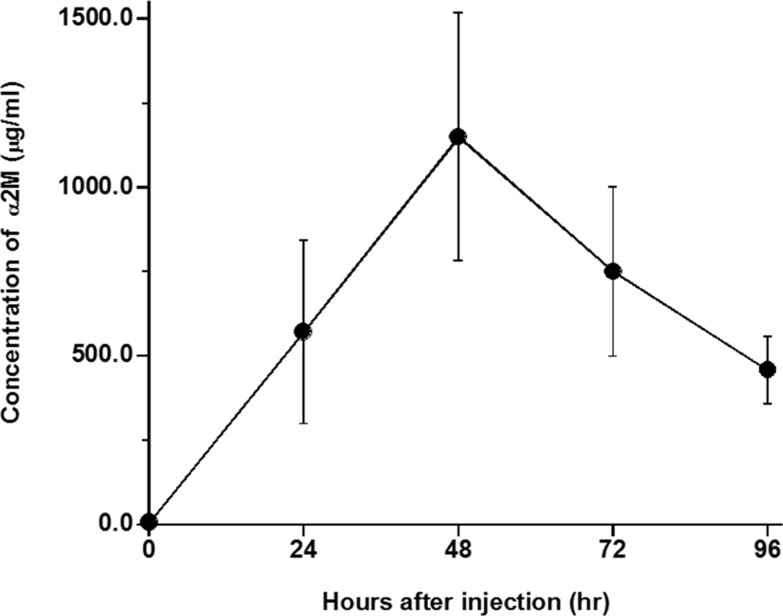
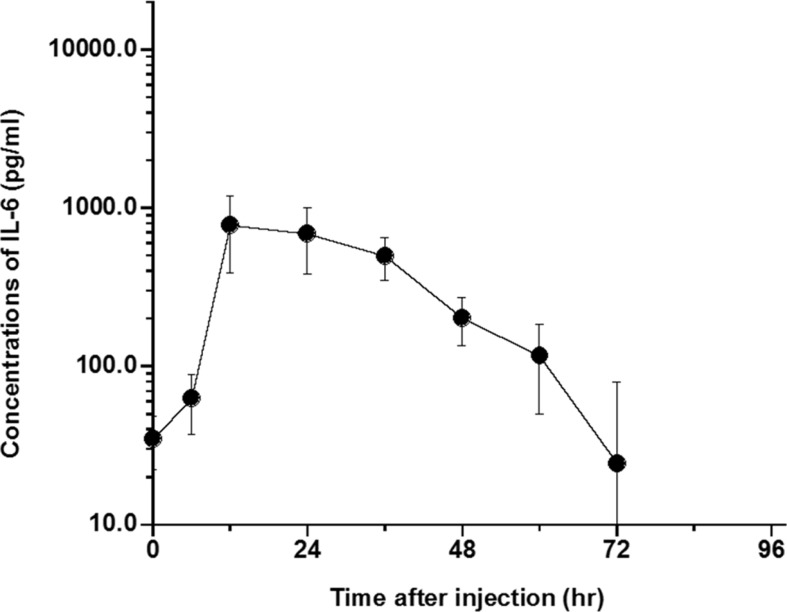
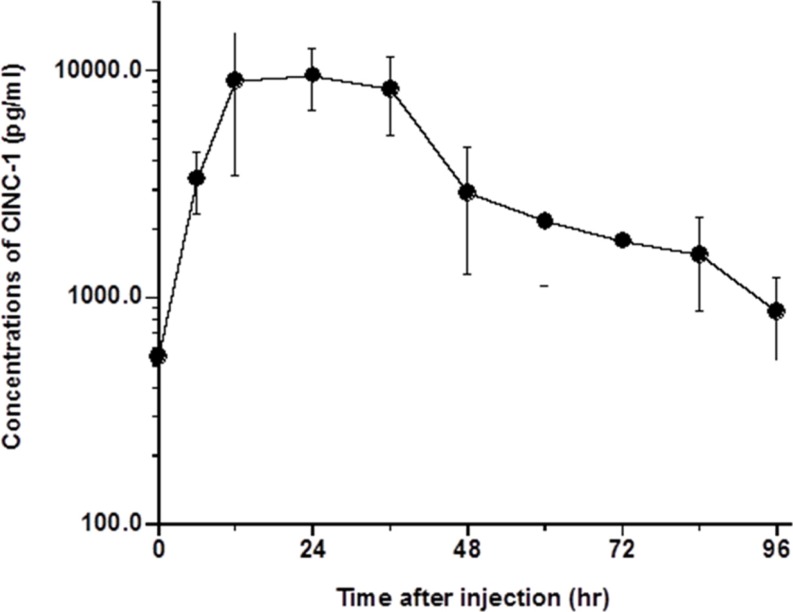
- The elimination half-lives of in Interleukin-6 (IL-6) and cytokine-induced neutrophil chemoattractant-1 (CINC-1) in rats after inflammatory stimulation were investigated. Five male Sprague-Dawley rats were used (age, 9 weeks; body weight, 235-375 g). Turpentine oil was intramuscularly injected at a dose of 2 mL/kg body weight to induce acute inflammation. Blood was collected pre-injection and 6, 12, 24, 36, 48, 60, 72, 84, and 96 h after the turpentine oil injection. Serum concentrations of IL-6, CINC-1, and α₂-macroglobulin (α2M) were measured by enzyme-linked immunosorbent assay. Half-lives were calculated as 0.693/elimination rate constant. The serum concentration of α2M peaked at 48 h after turpentine oil injection. Serum concentrations of IL-6 and CINC-1 increased and peaked at 12 and 24 h, respectively. The terminal elimination half-lives of IL-6 and CINC-1 were 15.5 and 29.9 h, respectively. The half-life of CINC-1 was significantly longer than that of IL-6 (P=0.006). These results suggested that these cytokines synthesized in response to inflammatory stimulation were rapidly eliminated in rats. The serum concentrations of these cytokines should be measured at an early stage if these cytokines will be used as surrogate inflammatory markers instead of acute-phase proteins.
MeSH Terms
Figure
Reference
-
1. Inoue S, Jinbo T, Shino M, Iguchi K, Nomura M, Kawato K, Yamamoto S. Determination of α2-macroglobulin concentrations in healthy rats of various ages and rats inoculated with turpentine oil by an enzyme-linked immunosorbent assay. J Exp Anim Sci. 2001; 42(1):44–49.2. Jinbo T, Motoki M, Yamamoto S. Variation of serum α2-macroglobulin concentration in healthy rats and rats inoculated with Staphylococcus aureus or subjected to surgery. Comp Med. 2001; 51(4):332–335. PMID: 11924791.3. Jinbo T, Sakamoto T, Yamamoto S. Serum α2-macroglobulin and cytokine measurements in an acute inflammation model in rats. Lab Anim. 2002; 36(2):153–157. PMID: 11943079.4. Honjo T, Kuribayashi T, Seita T, Mokonuma Y, Yamaga A, Yamazaki S, Yamamoto S. The effects of interleukin-6 and cytokine-induced neutrophil chemoattractant-1 on α2-macroglobulin production in rats. Exp Anim. 2010; 59(5):589–594. PMID: 21030786.5. Kuribayashi T, Tomizawa M, Seita T, Tagata K, Yamamoto S. Relationship between production of acute-phase proteins and strength of inflammatory stimulation in rats. Lab Anim. 2011; 45(3):215–218. PMID: 21669904.
Article6. Kuribayashi T, Seita T, Kawato K, Yamazaki S, Yamamoto S. Comparison of α2-macroglobulin synthesis by juvenile vs. mature rats after identical inflammatory stimulation. Inflammation. 2013; 36(6):1448–1452. PMID: 23856939.7. Honjo T, Kuribayashi T, Matsumoto M, Yamazaki S, Yamamoto S. Kinetics of α2-macroglobulin and α1-acid glycoprotein in rats subjected to repeated acute inflammatory stimulation. Lab Anim. 2010; 44(2):150–154. PMID: 19858170.8. Kuribayashi T, Seita T, Honjo T, Yamazaki S, Momotani E, Yamamoto S. Impairment of α2-macroglobulin synthesis in experimental hepatopathic rats treated with turpentine oil. Exp Anim. 2012; 61(2):125–130. PMID: 22531727.
Article9. Todd I, Spickett G. Lecture notes Immunology. 6th ed. USA: Wiley-Blackwell;2011. p. 42–54.10. Weber RL, Iacono VJ. The cytokines: a review of interleukins. Periodontal Clin Investig. 1997; 19(1):17–22.11. Whiteside TL. Cytokine assays. Biotechniques. 2002; 33:S4–S15.
Article12. Prasad VG, Vivek Ch, Anand Kumar P, Ravi Kumar P, Rao GS. Turpentine oil induced inflammation decreases absorption and increases distribution of phenacetion without altering its elimination process in rats. Eur J Drug Metab Pharmacokinet. 2015; 40(1):23–28. PMID: 24356809.13. Veilleux-Lemieux D, Castel A, Carrier D, Beaudry F, Vachon P. Pharmacokinetics of ketamine and xylazine in young and old Sprague-Dawley rats. J Am Assoc Lab Anim Sci. 2013; 52(5):567–570. PMID: 24041212.14. Rycroft D, Sosabowski J, Coulstock E, Davies M, Morrey J, Friel S, Kelly F, Hamatake R, Oveèka M, Prince R, Goodall L, Sepp A, Walker A. Pharmacokinetic characteristics, pharmacodynamic effect and in vivo antiviral efficacy of liver-targeted interferon alpha. PLoS One. 2015; 10(2):e0117847. PMID: 25689509.15. Kuribayashi T, Seita T, Momotani E, Yamazaki S, Hagimori K, Yamamoto S. Elimination half-lives of acute-phase proteins in rats and beagle dogs during acute inflammation. Inflammation. 2015; 38(4):1401–1405. PMID: 25633424.16. Hu X, Shang S, Nestorov I, Hasan J, Seddighzadeh A, Dawson K, Sperling B, Werneburg B. COMPARE: Pharmacokinetic profiles of subcutaneous peginterferon beta-1a and subcutaneous interferon beta-1a over 2 weeks in healthy subjects. Br J Clin Pharmacol. 2016; 82(2):380–388. PMID: 27060836.17. Grace MJ, Cutler D. Pegylating IFNs at His-34 improves the in vitro antiviral activity through the JAK/STAT pathway. Antivir Chem Chemother. 2004; 15(6):287–297. PMID: 15646642.18. Foster GR. Pegylated interferons for the treatment of chronic hepatitis C: pharmacological and clinical differences between peginterferon-alpha-2a and peginterferon-alpha-2b. Drugs. 2010; 70(2):147–165. PMID: 20108989.19. White JT, Newsome SD, Kieseier BC, Bermel RA, Cui Y, Seddighzadeh A, Hung S, Crossman M, Subramanyam M. Incidence, characterization, and clinical impact analysis of peginterferon beta1a immunogenicity in patients with multiple sclerosis in the ADVANCE trial. Ther Adv Neurol Disord. 2016; 9(4):239–249. PMID: 27366230.
Article20. Yamazaki N, Uhara H, Wada H, Matsuda K, Yamamoto K, Shimamoto T, Kiyohara Y. Phase I study of pegylated interferon-alpha-2a as an adjuvant therapy in Japanese patients with malignant melanoma. J Dermatol. 2016; 43(10):1146–1153. PMID: 27087489.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Neutrophil Elastase inhibitor, ICI 200,355, on Interleukin-1 Induced acute lung injury in rats
- The role of cytokines in rhinosinusitis
- The Efficacy of alpha-lipoic Acid on the Endotoxin-induced Acute Lung Injury
- The Central and Peripheral Production of Pro-inflammatory Cytokine, IL-1b after Immune and Stress Stimulation in Rats
- Xylitol Mitigate Neutrophil Inflammatory Response Against Porphyromonas gingivalis Infection