Lab Anim Res.
2018 Jun;34(2):65-74. 10.5625/lar.2018.34.2.65.
Effects of dietary lipid-coated zinc on the antioxidant defense system in the small intestine and liver of piglets
- Affiliations
-
- 1Department of Animal Science and Biotechnology, Gyeongnam National University of Science and Technology, Jinju, Korea. isjang@gntech.ac.kr
- 2Department of Animal Resources Technology, Gyeongnam National University of Science and Technology, Jinju, Korea.
- 3Regional Animal Research Center, Gyeongnam National University of Science and Technology, Jinju, Korea.
- KMID: 2413845
- DOI: http://doi.org/10.5625/lar.2018.34.2.65
Abstract
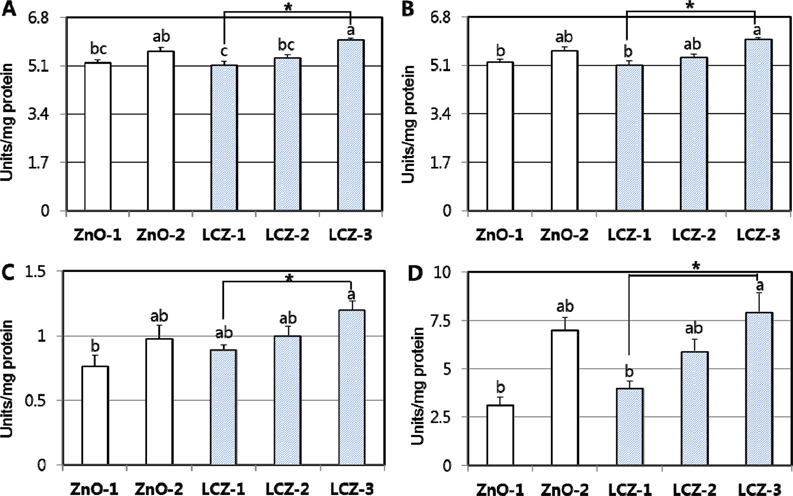
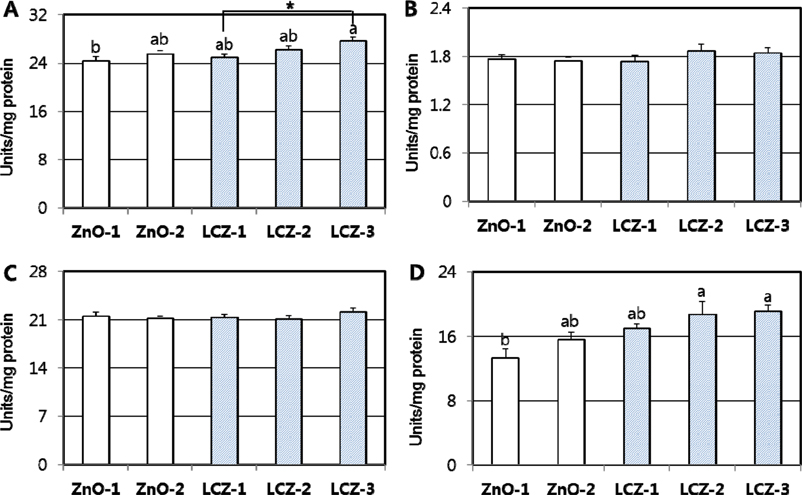
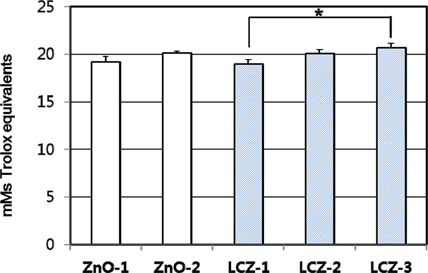
- The purpose of the study was to investigate the effects of lipid-coated ZnO (LCZ) and the level of LCZ compared with ordinary zinc oxide (ZnO) on antioxidant defense system in the intestine and liver of piglets. A total of forty piglets (n=8) were fed a diet supplemented with 100 ppm Zn with ZnO (ZnO-1), 2,500 ppm Zn with ZnO (ZnO-2), 100 ppm Zn as LCZ (LCZ-1), 200 ppm Zn as LCZ (LCZ-2), or 400 ppm Zn as LCZ (LCZ-3) for 14-d, respectively. The LCZ-3 group resulted in higher (P < 0.05) mRNA expressions and activities of CuZn-superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT), and glutathione S-transferase (GST) in jejunal mucosa compared with the ZnO-1 and LCZ-1 groups, while no difference was observed in the mRNA level of antioxidant genes between the ZnO-1 and ZnO-2 groups. Within the LCZ groups, the LCZ level linearly and quadratically (P < 0.01) increased antioxidant enzymes in the jejunum. The maximum response of jejunal antioxidant enzymes to Zn supplementation was achieved by 400 ppm of LCZ. Hepatic mRNA expression of antioxidant enzymes was unaffected by Zn source and level, while hepatic SOD and GST activities were greater (P < 0.05) in the LCZ-3 group than in the ZnO-1 group. No difference was observed in lipid peroxidation of the jejunum and liver and the total antioxidant power of plasma among groups. In conclusion, a supplementation with 400 ppm of LCZ resulted in a maximum increase in antioxidant enzymes, indicating that LCZ may affect antioxidant defense system more profoundly than ZnO.
Keyword
MeSH Terms
Figure
Reference
-
1. Prasad AS. Zinc: an antioxidant and anti-inflammatory agent: role of zinc in degenerative disorders of aging. J Trace Elem Med Biol. 2014; 28(4):364–371.
Article2. Wang MQ, Tao WJ, Ye SS, Du YJ, Wang C, Shen SX. Effects of dietary pharmacological zinc on growth, liver metallothionein, Cu, Zn-SOD concentration and serum parameters in piglets. J Anim Vet Adv. 2012; 11(9):1390–1394.
Article3. Prasad AS, Kucuk O. Zinc in cancer prevention. Cancer Metastasis Rev. 2002; 21(3-4):291–295.4. Sahin K, Sahin N, Kucuk O, Hayirli A, Prasad AS. Role of dietary zinc in heat-stressed poultry: a review. Poult Sci. 2009; 88(10):2176–2183.
Article5. Tupe RS, Tupe SG, Tarwadi KV, Agte VV. Effect of different dietary zinc levels on hepatic antioxidant and micronutrients indices under oxidative stress conditions. Metabolism. 2010; 59(11):1603–1611.
Article6. Bun SD, Guo YM, Guo FC, Ji FJ, Cao H. Influence of organic zinc supplementation on the antioxidant status and immune responses of broilers challenged with Eimeria tenella. Poult Sci. 2011; 90(6):1220–1226.
Article7. Barman S, Srinivasan K. Attenuation of oxidative stress and cardioprotective effects of zinc supplementation in experimental diabetic rats. Br J Nutr. 2017; 117(3):335–350.
Article8. Oteiza PL, Olin KL, Fraga CG, Keen CL. Oxidant defense systems in testes from zinc-deficient rats. Proc Soc Exp Biol Med. 1996; 213(1):85–91.
Article9. Jemai H, Messaoudi I, Chaouch A, Kerkeni A. Protective effect of zinc supplementation on blood antioxidant defense system in rats exposed to cadmium. J Trace Elem Med Biol. 2007; 21(4):269–273.
Article10. Katouli M, Melin L, Jensen-Waern M, Wallgren P, Möllby R. The effect of zinc oxide supplementation on the stability of the intestinal flora with special reference to composition of coliforms in weaned pigs. J Appl Microbiol. 1999; 87(4):564–573.
Article11. Hill GM, Mahan DC, Carter SD, Cromwell GL, Ewan RC, Harrold RL, Lewis AJ, Miller PS, Shurson GC, Veum TL. Effect of pharmacological concentrations of zinc oxide with or without the inclusion of an antibacterial agent on nursery pig performance. J Anim Sci. 2001; 79:934–941.
Article12. Hedemann MS, Jensen BB, Poulsen HD. Influence of dietary zinc and copper on digestive enzyme activity and intestinal morphology in weaned pigs. J Anim Sci. 2006; 84(12):3310–3320.13. Zhou X, Li Y, Li Z, Cao Y, Wang F, Li C. Effect of dietary zinc on morphological characteristics and apoptosis related gene expression in the small intestine of Bama miniature pigs. Acta Histochem. 2017; 119(3):235–243.
Article14. Pluske JR, Pethick DW, Hopwood DE, Hampson DJ. Nutritional influences on some major enteric bacterial diseases of pig. Nutr Res Rev. 2002; 15(2):333–371.
Article15. Hill GM. Minerals and mineral utilization in swine. In : Chiba LI, editor. Sustainable Swine Nutrition. Oxford: John Wiley & Sons, Inc.;2013. p. 173–195.16. Pekas JC. Zinc 65 metabolism: gastrointestinal secretion by the pig. Am J Physiol. 1966; 211(2):407–413.
Article17. Jang I, Kwon CH, Ha DM, Jung DY, Kang SY, Park MJ, Han JH, Park BC, Lee CY. Effects of a lipid-encapsulated zinc oxide supplement on growth performance and intestinal morphology and digestive enzyme activities in weanling pigs. J Anim Sci Technol. 2014; 56:29.
Article18. Kwon CH, Lee CY, Han SY, Kim SJ, Park BC, Jang I, Han JH. Effects of dietary supplementation of lipid-encapsulated zinc oxide on colibacillosis, growth and intestinal morphology in weaned piglets challenged with enterotoxigenic Escherichia coli. Anim Sci J. 2014; 85(8):805–813.19. Park BC, Jung DY, Kang SY, Ko YH, Ha DM, Kwon CH, Park MJ, Han JH, Jang I, Lee CY. Effects of dietary supplementation of a zinc oxide product encapsulated with lipid on growth performance, intestinal morphology, and digestive enzyme activities in weanling pigs. Anim Feed Sci Technol. 2015; 200:112–117.
Article20. Song YM, Kim MH, Kim HN, Jang I, Han JH, Fontamillas GA, Lee CY, Park BC. Effects of dietary supplementation of lipid-coated zinc oxide on intestinal mucosal morphology and expression of the genes associated with growth and immune function in weanling pigs. Asian-Australas J Anim Sci. 2018; 31(3):403–409.
Article21. Grüngreiff K, Reinhold D, Wedemeyer H. The role of zinc in liver cirrhosis. Ann Hepatol. 2016; 15(1):7–16.
Article22. Xia T, Lai W, Han M, Han M, Ma X, Zhang L. Dietary ZnO nanoparticles alters intestinal microbiota and inflammation response in weaned piglets. Oncotarget. 2017; 8(39):64878–64891.
Article23. Xu Z, Squires EJ, Bray TM. Effects of dietary zinc deficiency on the hepatic microsomal cytochrome P450 2B in rats. Can J Physiol Pharmacol. 1994; 72(3):211–216.
Article24. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001; 25(4):402–408.25. Kupfer D, Levin E. Monooxygenase drug metabolizing activity in CaCl2-aggregated hepatic microsomes from rat liver. Biochem Biophys Res Commun. 1972; 47(3):611–618.26. Tappel AL. Glutathione peroxidase and hydroperoxides. Methods Enzymol. 1978; 52:506–513.27. Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferase: The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974; 249(22):7130–7139.28. Melega S, Canistro D, De Nicola GR, Lazzeri L, Sapone A, Paolini M. Protective effect of Tuscan black cabbage sprout extract against serum lipid increase and perturbations of liver antioxidant and detoxifying enzymes in rats fed a high-fat diet. Br J Nutr. 2013; 110(6):988–997.
Article29. Bidlack WR, Tappel AL. Damage to microsomal membrane by lipid peroxidation. Lipids. 1973; 8(4):177–182.
Article30. Song Y, Leonard SW, Traber MG, Ho E. Zinc deficiency affects DNA damage, oxidative stress, antioxidant defenses, and DNA repair in rats. J Nutr. 2009; 139(9):1626–1631.
Article31. She Y, Huang Q, Li D, Piao X. Effects of proteinate complex zinc on growth performance, hepatic and splenic trace elements concentrations, antioxidative function and immune functions in weaned piglets. Asian-Australas J Anim Sci. 2017; 30(8):1160–1167.
Article32. Nijhoff WA, Peters WH. Induction of rat hepatic and intestinal glutathione S-transferases by dietary butyrated hydroxyanisole. Biochem Pharmacol. 1992; 44(3):596–600.33. de Waziers I, Boisset M, Atteba S. Pre- and postweaning development of drug-metabolizing enzyme activities in small intestine and liver of rats. Drug Metab Dispos. 1988; 16(2):310–315.34. Yang W, Chen Y, Cheng Y, Wen C, Zhou Y. Effects of zinc bearing palygorskite supplementation on the growth performance, hepatic mineral content, and antioxidant status of broilers at early age. Asian-Australas J Anim Sci. 2017; 30(7):1006–1012.
Article35. Uddin MG, Hossain MS, Rahman MA, Uddin AHMM, Bhuiyan MS. Elemental zinc is inversely associated with C-reactive protein and oxidative stress in chronic liver disease. Biol Trace Elem Res. 2017; 178(2):189–193.
Article36. Fridovich I. Superoxide dismutase. Enzymology. 1974; 41:36–40.
Article37. Matés JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000; 153(1-3):83–104.
Article38. Miroñczuk-Chodakowska I, Witkowska AM, Zujko ME. Endogenous non-enzymatic antioxidants in the human body. Adv Med Sci. 2018; 63(1):68–78.39. Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, Stiborova M, Adam V, Kizek R. The role of metallothionein in oxidative stress. Int J Mol Sci. 2013; 14(3):6044–6066.
Article40. Tang ZG, Chen GY, Li LF, Wen C, Wang T, Zhou YM. Effect of zinc-bearing zeolite clinoptilolite on growth performance, zinc accumulation, and gene expression of zinc transporters in broilers. J Anim Sci. 2015; 93(2):620–626.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Vitamin E Supplementation on Lipid Peroxide Levels of Blood and Liver in Zinc Deficient Rats
- Effect of dietary zinc deficiency on the enzymatic components of free radical defense system in the skin of rats
- The effects of coenzyme Qâ‚â‚€ supplement on blood lipid indices and hepatic antioxidant defense system in SD rats fed a high cholesterol diet
- Grape skin improves antioxidant capacity in rats fed a high fat diet
- Effects of Dietary Protein and Calcium Levels on Iron and Zine Balance in Young Korean Women