Nutr Res Pract.
2009 Dec;3(4):279-285.
Grape skin improves antioxidant capacity in rats fed a high fat diet
- Affiliations
-
- 1Department of Food and Nutrition, Yeungnam University, 214-1 Dae-dong, Gyeongsan-si, Gyeongbuk 712-749, Korea. Jsseo@ynu.ac.kr
Abstract
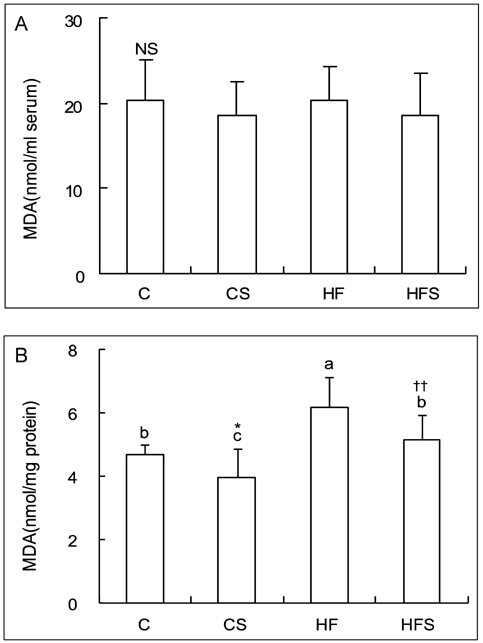
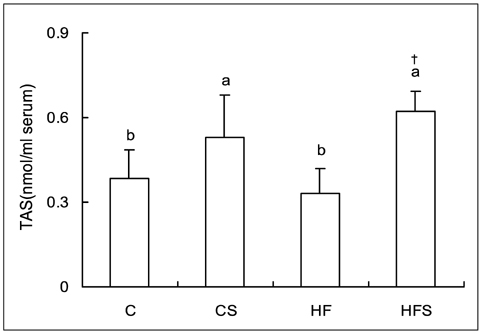
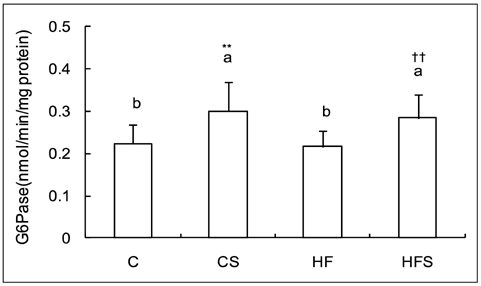
- This study was conducted to investigate the effect of dietary grape skin on lipid peroxidation and antioxidant defense system in rats fed high fat diet. The Sprague-Dawley rats were fed either control (5% fat) diet or high fat (25% fat) diet which was based on AIN-93 diet for 2 weeks, and then they were grouped as control group (C), control + 5% grape skin group (CS), high-fat group (HF), high fat + 5% grape skin group (HFS) with 10 rats each and fed corresponding diets for 4 weeks. The hepatic thiobarbituric acid reacting substances (TBARS) were increased in high fat group as compared with control group, but reduced by grape skin. The serum total antioxidant status, and activities of hepatic catalase and superoxide dismutase, xanthine oxidase and glucose-6-phosphatase were increased by supplementation of grape skin. Glutathione peroxidase activity was significantly higher in CS group than in C group. Grape skin feeding tended to increase the concentration of total glutathione, especially in control group. The ratio of reduced glutathione to oxidized glutathione was lower in high fat groups than in control groups. The ratio was increased by dietary supplementation of grape skin in control group. These results suggest that dietary supplementation of grape skin would be effective on protection of oxidative damage by lipid peroxidation through improvement of antioxidant defense system in rats fed high fat diet as well as rats with low fat diet.
Keyword
MeSH Terms
-
Animals
Catalase
Diet
Diet, High-Fat
Dietary Supplements
Glucose-6-Phosphatase
Glutathione
Glutathione Disulfide
Glutathione Peroxidase
Lipid Peroxidation
Obesity
Rats
Rats, Sprague-Dawley
Skin
Superoxide Dismutase
Thiobarbiturates
Vitis
Xanthine Oxidase
Catalase
Glucose-6-Phosphatase
Glutathione
Glutathione Disulfide
Glutathione Peroxidase
Superoxide Dismutase
Thiobarbiturates
Xanthine Oxidase
Figure
Reference
-
1. Acquaviva R, Russo A, Galvano F, Galvano G, Barcellona ML, Bolti GL, Vanella A. Cyanidin and cyanidin 3-O-β-D-g lucoside as DNA cleavage protectors and antioxidants. Cell Biol Toxicol. 2003. 19:243–252.
Article2. Aebi H. Bergmeyer HU, editor. Catalase. Methods of Enzymatic Analysis. 1974. 11. New York. USA: Academic Press;673–684.
Article3. Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004. 24:2783–2840.4. Akhileshwar V, Patel SP, Katyare SS. Diabetic cardiomyopathy and reactive oxygen species related parameters in male and female rats: A comparative study. Indian J Clin Biochem. 2007. 22:84–90.
Article5. Amirkhizi F, Siassi F, Minaie S, Djalali M, Rahimi A, Chamari M. Is obesity associated with increased plasma lipid peroxidation and oxidative stress in women? ARYA Atherosclerosis Journal. 2007. 2:189–192.6. Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985. 113:548–555.7. Bae HS. Effect of grape skin with resveratrol emplification on lipid metabolism and antioxidative system in rats fed high cholesterol diet. 2008. Gyeongbuk. Republic of Korea: Yeungnam University;MS thesis.8. Baginski ES, Foa PP, Zak B. Bergmeyer HU, editor. Glucose-6-phosphatase. Methods of Enzymatic Analysis. 1983. 2. New York. USA: Academic Press;876–880.
Article9. Belleville J. The Franch paradox: Possible involvemenet of ethanol in the protective effect against cardiovascular disease. Nutrition. 2002. 18:173–177.
Article10. Beltowski J, Wojcicka G, Gorny D, Marciniak A. The effect of dietary-induced obesity on lipid peroxidation, antioxidant enzymes and total plasma antioxidant capacity. J Physiol Pharmacol. 2000. 51:883–896.11. Bralley EE, Hargrove JL, Greenspan P, Hartle DK. Topical anti-inflammatory activities of Vitis rotundifolia(Muscadine grape) extracts in the tetradecanoylphorbol acetate model of ear inflammation. J Med Food. 2007. 10:636–642.
Article12. Bray GA, Paeratakul S, Popkin BM. Dietary fat and obetisy: a review of animal, clinical and epidemiological studies. Physiology & Behavior. 2004. 83:549–555.
Article13. Caballero B. The global epidemic of obesity: An overview. Epidemiol Rev. 2007. 29:1–5.
Article14. Campolo J, Maria RD, Caruso R, Accinni R, Turazza F, Parolini M, Roubina E, Chiara BD, Cighetti G, Frigerio M, Vitali E, Parodi O. Blood glutathone as independent marker of lipid peroxidation in heart failure. Int J Cardiol. 2006. 117:45–50.
Article15. Cho YJ, Kim JE, Chun HS, Kim CT, Kim SS, Kim CJ. Contents of resveratrol in different parts of grapes. Korean Journal of Food Science and Technoloy. 2003. 35:306–308.16. Cui J, Juhasz B, Tosaki A, Maulik N, Das DK. Cardioprotection with grape. J Cardiovasc Pharmacol. 2002. 40:762–769.17. Decorde K, Teissedre PL, Sutra T, Ventura E, Cristol JP, Rouanet JM. Chardonnay grape seed procyanidin extract supplementation prevents high-fat diet-induced obesity in hamsters by improving adipokine imbalance and oxidative stress markers. Mol Nutr Food Res. 2009. 53:659–666.
Article18. Delmas D, Jannin B, Latruffe N. Resveratrol: Preventing properties against vascular alterations and ageing. Mol Nutr Food Res. 2005. 49:377–395.
Article19. Evans P, Halliwell B. Micronutrients: Oxidabt/antioxidant status. Br J Nutr. 2001. 85:S67–S74.20. Falchi M, Bertelli A, Scalzo R, Morassut M, Morelli R, Das S, Cui J, Das DK. Comparison of cardioprotective abilities between the flesh and skin of grapes. J Agric Food Chem. 2006. 54:6613–6622.
Article21. Fang YZ, Yang S, Wu G. Free radicals, antioxidants and nutrition. Nutrition. 2002. 18:872–879.
Article22. STAT database. FAO. 2005. Access on 7/20/2007. www.Fao.org.23. Feillet-Coudray C, Sutra T, Fouret G, Ramos J, Wrutniak-Cabello C, Cabello G, Cristol JP, Coudray C. Oxidative stress in rats fed a high-fat high-sucrose diet and preventive effect of polyphenols: Involvement of mitochondrial and NAD(P)H oxidase systems. Free Radic Biol Med. 2009. 46:624–632.
Article24. Frederiksen H, Mortensen A, Schroder M, Frandsen H, Bysted A, Kunthsen P, Rasmussen SE. Effects of red grape skin and seed extract supplementation on atherosclerosis in watanabe heritable hyperlipidemic rabbits. Mol Nutr Food Res. 2007. 51:564–571.
Article25. Fukao H, Ijiri Y, Miura M, Hashimoto M, Yamashita T, Fukunaga C, Oiwa K, Kawai Y, Suwa M, Yamamoto J. Effect of trans-resveratrol on the thrombogenicity and atherogenicity in apolipoprotein E-deficient and low-density lipoprotein receptor-deficient mice. Blood Coagul Fibrinolysis. 2004. 15:441–446.
Article26. Furukawa S, Fujita T, Shimabukuro M, Lwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004. 114:1752–1761.
Article27. Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferase. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974. 249(22):7130–7139.28. Hogeboom GH. Fractionation of cell components of animal tissues. Meth Enzymol. 1955. 1:16–19.29. Hsu CL, Yen GC. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br J Nutr. 2007. 98:727–735.
Article30. Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, Gharbi N, Kamoun A, El-Fazaa S. Protective effect of resveratrol on ethanol-induced lipid peroxidation in rats. Alcohol Alcohol. 2006. 41:236–239.
Article31. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951. 193:265–275.
Article32. Madeira SV, Auger C, Anselm E, Chataigneau M, Chataigneau T, Moura RS, Schini-Kerth VB. eNOS activation induced by a polyphenol-rich grape skin extract in porcine coronary arteries. J Vasc Res. 2009. 46:406–416.
Article33. Makris DP, Boskou G, Andrikopoulos NK. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J Food Compost Anal. 2007. 20:125–132.
Article34. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974. 47:469–474.
Article35. Miller NJ, Rice0Ebans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci. 1993. 84:407–412.
Article36. Miura D, Miura Y, Yagasaki K. Hypolipidemic action of dietary resveratrol, a phytoalexin in grapes and red wine, in hepatoma-bearing rats. Life Sci. 2003. 73:1393–1400.
Article37. Negro C, Tommasi L, Miceli A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour Technol. 2003. 87:41–44.
Article38. Oben JE, Enyegue DM, Fomekong GI, Soukontoua YB, Agbor GA. The effect of Cissus quadrangularis (GQR-300) and a Cissus formulation (CORE) on obesity and obesity-induced oxidative stress. Lipids Health Dis. 2007. 6:4–12.39. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979. 95:351–358.
Article40. Olusi SO. Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. Int J Obes. 2002. 26:1159–1164.
Article41. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967. 70:158–169.42. Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004. 7:97–110.
Article43. Ramirez-Tortosa C, Andersen OM, Gardner PT, Morrice PC, Wood SG, Duthie SJ, Collins AR, Duthie GG. Anthocyanin-rich extract decreases indices of lipid peroxidation and DNA damage in vitamin E-depleted rats. Free Radic Biol Med. 2001. 31:1033–1037.
Article44. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999. 26:1231–1237.
Article45. Rocha KK, Souza GA, Ebaid GX, Seiva FRF, Cataneo AC, Novelli ELB. Resveratrol toxicity: Effects on risk factors for atherosclerosis and hepatic oxidatice stress in standard an high-fat diets. Food Chem Toxicol. 2009. 47:1362–1367.
Article46. Scarlatti F, Sala G, Somenzi G, Signorelli P, Sacchi N, Ghidoni R. Resveratrol induces growth inhibition and apoptosis in metastatic breast cancer cells via de novo ceramide signaling. FASEB J. 2003. 17:2339–2341.
Article47. Schneider Y, Vincent F, Duranton B, Badolo L, Gosse F, Bergmann C, Seiler N, Raul F. Anti-proliferative effect of resveratrol, a natural component of grapes and wine, on human colonic cancer cells. Cancer Lett. 2000. 158:85–91.
Article48. Sener G, Topaloglu N, Sehirli O, Ercan F, Gedik N. Resveratrol alleviates bleomycin-induced lung injury in rats. Pulm Pharmacol Ther. 2007. 20:642–649.
Article49. Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999. 27:916–921.
Article50. Singleton VL, Joseph A, Possi JR. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965. 16:144–158.51. Strip F, della Corte E. The regulation of rat liver xanthine oxidase; Conversion in vitro of the enzyme activity from dehydrogenase (Type D) to oxidase (Type O). J Biol Chem. 1969. 244:3855–3863.52. Su H, Hung L, Chen J. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2006. 290:E1339–E1346.
Article53. Tsuda T, Horio F, Uchida K, Aoki H, Osawa T. Dietary cyanidin 3-O-β-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr. 2003. 133:2125–2130.
Article54. Vasdev S, Gill V. Antioxidants in the treatment of hypertension. Int J Angiol. 2005. 14:60–73.
Article55. West DB, York B. Dietary fat, genetic predisposition, and obesity: lessons from animal models. Am J Clin Nutr. 1998. 67:505S–512S.
Article56. Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976. 15:212–216.
Article57. Yassa N, Razavi BH, Hadjiakhoondi A. Free radical scavenging and lipid peroxidation activity of the shahani black grape. Pak J Biol Sci. 2008. 11:2513–2516.
Article58. Young JF, Dragsted LO, Daneshvar B, Lauridsen ST, Hansen M, Sandstrom B. The effect of grape skin extract on oxidative status. Br J Nutr. 2000. 84:505–513.
Article59. Yunoki K, Sasaki G, Tokuji Y, Kinoshita M, Naito A, Aida K, Ohnishi M. Effect of dietary wine pomace eztract and oleanolic acid on plasma lipids in rats fed high-fat diet and its DNA microarray analysis. J Agric Food Chem. 2008. 56:12052–12058.
Article60. Zhang X, Dong F, Ren J, Driscoll MJ, Culver B. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol. 2005. 191:318–325.
Article61. Zhang Y, Liu Y, Wang T, Li B, Li H, Wang Z, Yang B. Resveratrol, a natural ingredient of grape skin: Antiarrhythmic efficacy and ionic mechanisms. Biochem Biophys Res Commun. 2006. 340:1192–1199.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Dietary Grape Pomace on Lipid Oxidation and Related Enzyme Activities in Rats Fed High Fat Diet
- The Effect of Grape Seed Oil, Perilla Oil, or Corn Oil-Containing Diet on Lipid Patterns in Rats and Fatty-Acid Composition in Their Liver Tissues
- Suppression of oxidative stress by grape seed supplementation in rats
- Antioxidant effects of fucoxanthin rich powder in rats fed with high fat diet
- The effects of Angelica keiskei Koidz on the expression of antioxidant enzymes related to lipid profiles in rats fed a high fat diet