Nutr Res Pract.
2012 Feb;6(1):9-15.
The effects of Angelica keiskei Koidz on the expression of antioxidant enzymes related to lipid profiles in rats fed a high fat diet
- Affiliations
-
- 1Korea Food Research Institute, 516 Baekhyun-dong, Bundang-gu, Sungnam-si, Gyeonggi 463-746, Korea. kem@kfri.re.kr
- 2College of Biotechnology, Youngnam University, Gyeongbuk 712-749, Korea.
- 3Pulmuone.Co., Ltd, Seoul 120-749, Korea.
Abstract
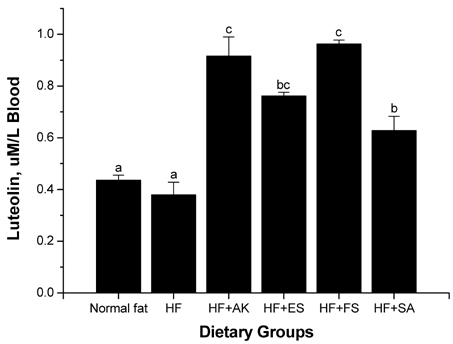
- This study was performed to examine the feeding effects of Angelica keiskei Koidz (AK) and its processed products on serum, liver, and body fat content and the expression of antioxidant genes in rats fed a high fat diet. AK and its processed products were added at 3-5% to a high fat diet and fed to adult rats for 6 weeks. In experiment 1 (EXP 1), the rats were fed with one of six diets including a control diet (normal fat), high fat diet (HF), and HF + AK additives groups (four groups). In experiment 2 (EXP 2), the rats were separated into three groups of HF, HF + AK whole leaves, and HF + fermented juice (FS) + squeeze (SA). Body weight was not different among the groups in either experiment. The liver weight was lower in the FS and SA groups compared to that in the other groups (P < 0.05). Serum luteolin was higher in the AK and processed products groups compared to that in the HF group (P < 0.05). Gene expression of the antioxidative enzymes catalase and glutathione-s-reductase in the liver was higher in the AK processed products group than that in the other groups (P < 0.05). The results suggest that the intake of AK and its processed products increased the expression of antioxidant enzymes in animals fed a high fat diet, reduced hepatic cholesterol content, and increased the effective absorption of luteolin.
MeSH Terms
Figure
Reference
-
1. Kozawa M, Morita N, Baba K, Hata K. Chemical components of the roots of Angelica keiskei Koidzumi. II. The structure of the chalcone derivatives. Yakugaku Zasshi. 1978. 98:210–214.
Article2. Kim OK, Kung SS, Park WB, Lee MW, Ham SS. The nutritional components of aerial whole plant and juice of Angelica keiskei Koidz. Korean J Food Sci Technol. 1992. 24:592–596.3. Mitsuhashi H. Illustrated Medicinal Plants of the World in Colour. 1988. Tokyo: Hokuryukan;350.4. Eyre H, Kahn R, Robertson RM. ACS/ADA/AHA Collaborative Writing Committee. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. CA Cancer J Clin. 2004. 54:190–207.
Article5. Hirayama T. Knudsen I, editor. Nutrition and cancer - a large scale cohort study. Genetic Toxicology of the Diet. 1986. New York: Liss Inc;94.6. Neuhouser ML. Dietary flavonoids and cancer risk: evidence from human population studies. Nutr Cancer. 2004. 50:1–7.
Article7. Mojzisová G, Kuchta M. Dietary flavonoids and risk of coronary heart disease. Physiol Res. 2001. 50:529–535.8. Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002. 22:19–34.
Article9. Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther. 2001. 90:157–177.
Article10. López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem. 2009. 9:31–59.
Article11. Kim SH, Shin KJ, Kim D, Kim YH, Han MS, Lee TG, Kim E, Ryu SH, Suh PG. Luteolin inhibits the nuclear factor-kappa B transcriptional activity in Rat-1 fibroblasts. Biochem Pharmacol. 2003. 66:955–963.
Article12. Kotanidou A, Xagorari A, Bagli E, Kitsanta P, Fotsis T, Papapetropoulos A, Roussos C. Luteolin reduces lipopolysaccharide-induced lethal toxicity and expression of proinflammatory molecules in mice. Am J Respir Crit Care Med. 2002. 165:818–823.
Article13. Gates MA, Tworoger SS, Hecht JL, De Vivo I, Rosner B, Hankinson SE. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int J Cancer. 2007. 121:2225–2232.
Article14. Zhu X, Zhang H, Lo R. Phenolic compounds from the leaf extract of artichoke (Cynara scolymus L.) and their antimicrobial activities. J Agric Food Chem. 2004. 52:7272–7278.
Article15. Ichimura T, Yamanaka A, Ichiba T, Toyokawa T, Kamada Y, Tamamura T, Maruyama S. Antihypertensive effect of an extract of Passiflora edulis rind in spontaneously hypertensive rats. Biosci Biotechnol Biochem. 2006. 70:718–721.
Article16. Institute of Laboratory Animal Resources. Guide for the Care and Use of Laboratory Animals. 1996. Washington, D.C.: National Academy Press;21–55.17. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972. 18:499–502.
Article18. Shimoi K, Okada H, Furugori M, Goda T, Takase S, Suzuki M, Hara Y, Yamamoto H, Kinae N. Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans. FEBS Lett. 1998. 438:220–224.
Article19. Zhou P, Li LP, Luo SQ, Jiang HD, Zeng S. Intestinal absorption of luteolin from peanut hull extract is more efficient than that from individual pure luteolin. J Agric Food Chem. 2008. 56:296–300.
Article20. King RA, Broadbent JL, Head RJ. Absorption and excretion of the soy isoflavone genistein in rats. J Nutr. 1996. 126:176–182.
Article21. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957. 226:497–509.
Article22. Le Lay S, Lefrère I, Trautwein C, Dugail I, Krief S. Insulin and sterol-regulatory element-binding protein-1c (SREBP-1C) regulation of gene expression in 3T3-L1 adipocytes. Identification of CCAAT/enhancer-binding protein beta as an SREBP-1C target. J Biol Chem. 2002. 277:35625–35634.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Freeze-dried Green Vegetable Extract on Lipid Profiles and Antioxidant Status in the Rat
- Effect of Dietary Grape Pomace on Lipid Oxidation and Related Enzyme Activities in Rats Fed High Fat Diet
- Effects of Isoflavone Supplementation on Lipid Profiles and Antioxidant Enzyme Activities in Growing Rats Fed High Fat Diet
- Effects of Soyoligosaccharide on Lipid Metabolism in Rats Fed the High Fat or Low Fat Diet
- Grape skin improves antioxidant capacity in rats fed a high fat diet