Korean J Radiol.
2018 Aug;19(4):767-776. 10.3348/kjr.2018.19.4.767.
Migraine with Aura: Surface-Based Analysis of the Cerebral Cortex with Magnetic Resonance Imaging
- Affiliations
-
- 1Faculty of Medicine, University of Belgrade, Belgrade 11000, Serbia. ip7med@yahoo.com
- 2Faculty of Physical Chemistry, University of Belgrade, Belgrade 11000, Serbia.
- 3Department of Radiology, Special Hospital for Prevention and Treatment of Cerebrovascular Diseases “Saint Savaâ€, Belgrade 11000, Serbia.
- 4Center for Headaches, Neurology Clinic, Clinical Center of Serbia, Belgrade 11000, Serbia.
- KMID: 2413706
- DOI: http://doi.org/10.3348/kjr.2018.19.4.767
Abstract
OBJECTIVE
Previous migraine studies have reported gray matter alterations in various cortical regions with conflicting results. This study aimed to explore a cortical morphometric difference in migraineurs with aura (MA) compared to healthy subjects (HS) and to delineate a possible difference between the cortical morphological features and different aura phenotypes.
MATERIALS AND METHODS
Forty-eight MA and 30 HS that were balanced by sex, age, and educational level were selected for this study. T2-weighted and three-dimensional T1-weighted magnetic resonance imaging (MRI) of the brain were acquired using a 1.5T MRI scanner. Surface-based morphometry from the MRI data was used to identify differences between the MA and HS group, and then between MA subgroups. The MA group was subdivided into migraineurs who experienced only visual aura (MVA) and migraineurs who had visual, somatosensory and dysphasic symptoms (MVA+).
RESULTS
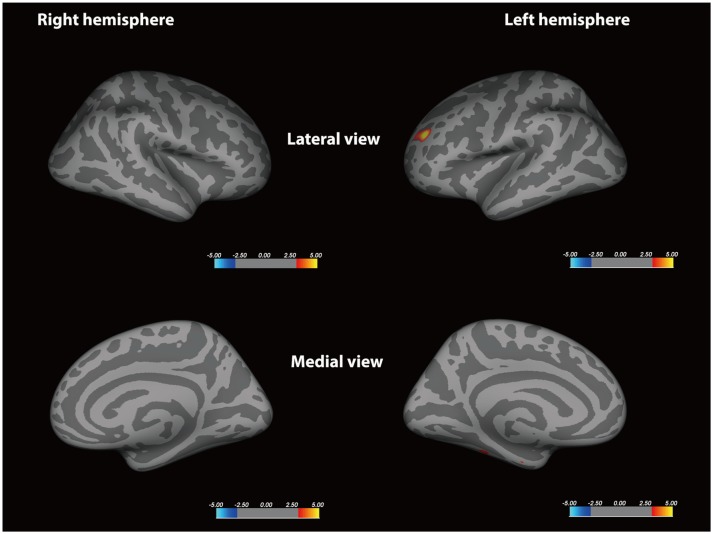
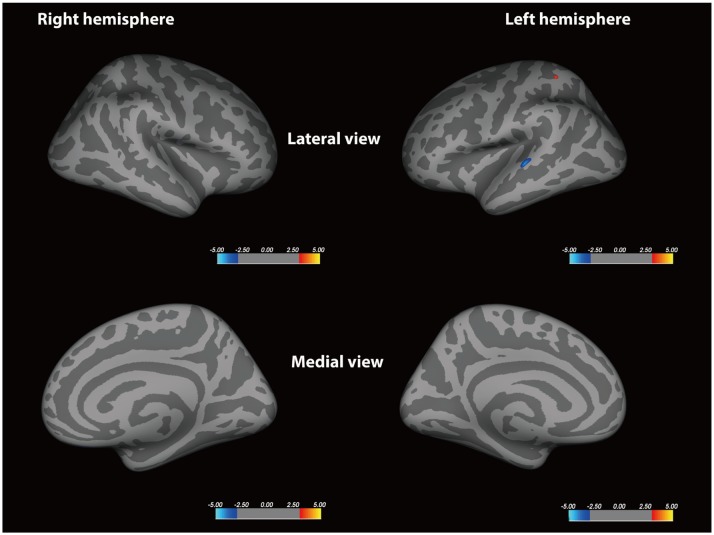
The MVA+ group had significantly reduced cortical surface area of the left rostral middle frontal cortex compared with the MVA group (p < 0.001). Migraine patients had significantly reduced volume of the left fusiform gyrus relative to HS (p < 0.001). Also, the sulcal depth increased at the level of the left temporal pole in the MVA+ group relative to the MVA group (p < 0.001). The vertex-by-vertex analysis did not exhibit any significant difference in cortical thickness between MA and HS, and between MVA+ and MVA, when corrected for multiple comparisons.
CONCLUSION
Migraineurs with aura demonstrates different morphometric features from HS in multiple cortical regions. MVA+ have different morphometric features in the left frontal and temporal lobe relative to MVA, which could be a source of distinct symptoms and serve as potential biomarkers of different MA subtypes.
Keyword
MeSH Terms
Figure
Reference
-
1. Stovner Lj, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007; 27:193–210. PMID: 17381554.
Article2. Vincent MB, Hadjikhani N. Migraine aura and related phenomena: beyond scotomata and scintillations. Cephalalgia. 2007; 27:1368–1377. PMID: 17944958.
Article3. Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001; 98:4687–4692. PMID: 11287655.
Article4. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013; 33:629–808. PMID: 23771276.5. Petrusic I, Zidverc-Trajkovic J, Podgorac A, Sternic N. Underestimated phenomena: higher cortical dysfunctions during migraine aura. Cephalalgia. 2013; 33:861–867. PMID: 23430982.
Article6. Petrusic I, Podgorac A, Zidverc-Trajkovic J, Radojicic A, Jovanovic Z, Sternic N. Do interictal microembolic signals play a role in higher cortical dysfunction during migraine aura? Cephalalgia. 2016; 36:561–567. PMID: 26419790.
Article7. Kim JH, Suh SI, Seol HY, Oh K, Seo WK, Yu SW, et al. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008; 28:598–604. PMID: 18422725.
Article8. DaSilva AF, Granziera C, Snyder J, Hadjikhani N. Thickening in the somatosensory cortex of patients with migraine. Neurology. 2007; 69:1990–1995. PMID: 18025393.
Article9. Hougaard A, Amin FM, Larsson HB, Rostrup E, Ashina M. Increased intrinsic brain connectivity between pons and somatosensory cortex during attacks of migraine with aura. Hum Brain Mapp. 2017; 38:2635–2642. PMID: 28240389.
Article10. Datta R, Aguirre GK, Hu S, Detre JA, Cucchiara B. Interictal cortical hyperresponsiveness in migraine is directly related to the presence of aura. Cephalalgia. 2013; 33:365–374. PMID: 23359872.
Article11. Messina R, Rocca MA, Colombo B, Valsasina P, Horsfield MA, Copetti M, et al. Cortical abnormalities in patients with migraine: a surface-based analysis. Radiology. 2013; 268:170–180. PMID: 23533286.
Article12. Valfré W, Rainero I, Bergui M, Pinessi L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache. 2008; 48:109–117. PMID: 18184293.
Article13. Hougaard A, Amin FM, Arngrim N, Vlachou M, Larsen VA, Larsson HB, et al. Sensory migraine aura is not associated with structural grey matter abnormalities. Neuroimage Clin. 2016; 11:322–327. PMID: 27298761.
Article14. Datta R, Detre JA, Aguirre GK, Cucchiara B. Absence of changes in cortical thickness in patients with migraine. Cephalalgia. 2011; 31:1452–1458. PMID: 21911412.
Article15. Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D. Concurrent functional and structural cortical alterations in migraine. Cephalalgia. 2012; 32:607–620. PMID: 22623760.
Article16. Granziera C, DaSilva AF, Snyder J, Tuch DS, Hadjikhani N. Anatomical alterations of the visual motion processing network in migraine with and without aura. PLoS Med. 2006; 3:e402. PMID: 17048979.
Article17. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999; 9:179–194. PMID: 9931268.19. Schmitz N, Admiraal-Behloul F, Arkink EB, Kruit MC, Schoonman GG, Ferrari MD, et al. Attack frequency and disease duration as indicators for brain damage in migraine. Headache. 2008; 48:1044–1055. PMID: 18479421.
Article20. Roberts DJ, Woollams AM, Kim E, Beeson PM, Rapcsak SZ, Lambon Ralph MA. Efficient visual object and word recognition relies on high spatial frequency coding in the left posterior fusiform gyrus: evidence from a case-series of patients with ventral occipito-temporal cortex damage. Cereb Cortex. 2013; 23:2568–2580. PMID: 22923086.
Article21. Barton JJ, Press DZ, Keenan JP, O'Connor M. Lesions of the fusiform face area impair perception of facial configuration in prosopagnosia. Neurology. 2002; 58:71–78. PMID: 11781408.
Article22. Banissy MJ, Stewart L, Muggleton NG, Griffiths TD, Walsh VY, Ward J, et al. Grapheme-color and tone-color synesthesia is associated with structural brain changes in visual regions implicated in color, form, and motion. Cogn Neurosci. 2012; 3:29–35. PMID: 24168647.
Article23. Coppola G, Di Renzo A, Tinelli E, Iacovelli E, Lepre C, Di Lorenzo C, et al. Evidence for brain morphometric changes during the migraine cycle: a magnetic resonance-based morphometry study. Cephalalgia. 2015; 35:783–791. PMID: 25414472.
Article24. Schwedt TJ, Chong CD, Chiang CC, Baxter L, Schlaggar BL, Dodick DW. Enhanced pain-induced activity of pain-processing regions in a case-control study of episodic migraine. Cephalalgia. 2014; 34:947–958. PMID: 24627432.
Article26. Petrusic I, Zidverc-Trajkovic J. Cortical spreading depression: origins and paths as inferred from the sequence of events during migraine aura. Funct Neurol. 2014; 29:207–212. PMID: 25473742.27. Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009; 19:2728–2735. PMID: 19299253.
Article28. Rocca MA, Messina R, Colombo B, Falini A, Comi G, Filippi M. Structural brain MRI abnormalities in pediatric patients with migraine. J Neurol. 2014; 261:350–357. PMID: 24305994.
Article29. White T, Andreasen NC, Nopoulos P, Magnotta V. Gyrification abnormalities in childhood- and adolescent-onset schizophrenia. Biol Psychiatry. 2003; 54:418–426. PMID: 12915286.
Article30. Deng F, Jiang X, Zhu D, Zhang T, Li K, Guo L, et al. A functional model of cortical gyri and sulci. Brain Struct Funct. 2014; 219:1473–1491. PMID: 23689502.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Characteristics of Migraine with Aura in Korean: a Clinic Based Study
- Are there network differences between the ipsilateral and contralateral hemispheres of pain in patients with episodic migraine without aura?
- Giant Arachnoid Granulations in Headache Mimicking Migraine with Aura
- White Matter Abnormalities of Migraine and Tension Type Headache in Young Patients Without Vascular Risk Factors
- Tc-99m HM-PAO SPECT in migraine between attacks