Int J Stem Cells.
2018 Jun;11(1):68-77. 10.15283/ijsc17052.
Therapeutic Efficacy of Mesenchymal Stem Cells and Mesenchymal Stem Cells-derived Neural Progenitors in Experimental Autoimmune Encephalomyelitis
- Affiliations
-
- 1Department of Immunology, Shiraz University of Medical Sciences, Shiraz, Iran. immunol2@gmail.com
- 2Student Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
- 3Autoimmune Diseases Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
- KMID: 2413502
- DOI: http://doi.org/10.15283/ijsc17052
Abstract
- BACKGROUND AND OBJECTIVES
The goal of treatment for MS is to reduce the inflammation and induce the regeneration of degenerated axons. Considering the anti-inflammatory and regenerative capacity of mesenchymal stem cell (MSCs), in this study the therapeutic efficacy of allogeneic MSCs and MSCs-derived neural progenitor cells (MSCs-NPs) was investigated in cellular therapy of chronic experimental autoimmune encephalomyelitis (EAE).
METHODS AND RESULTS
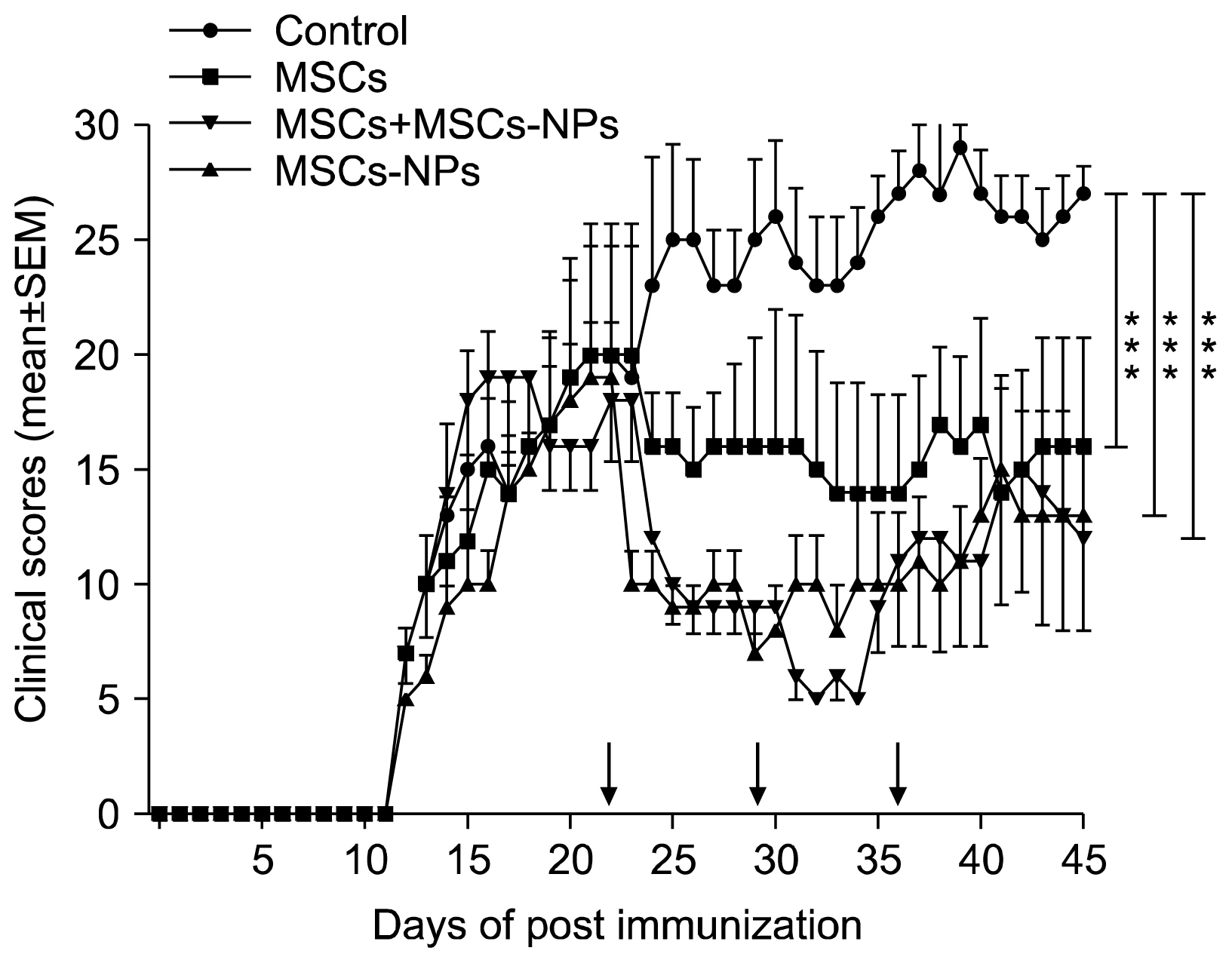
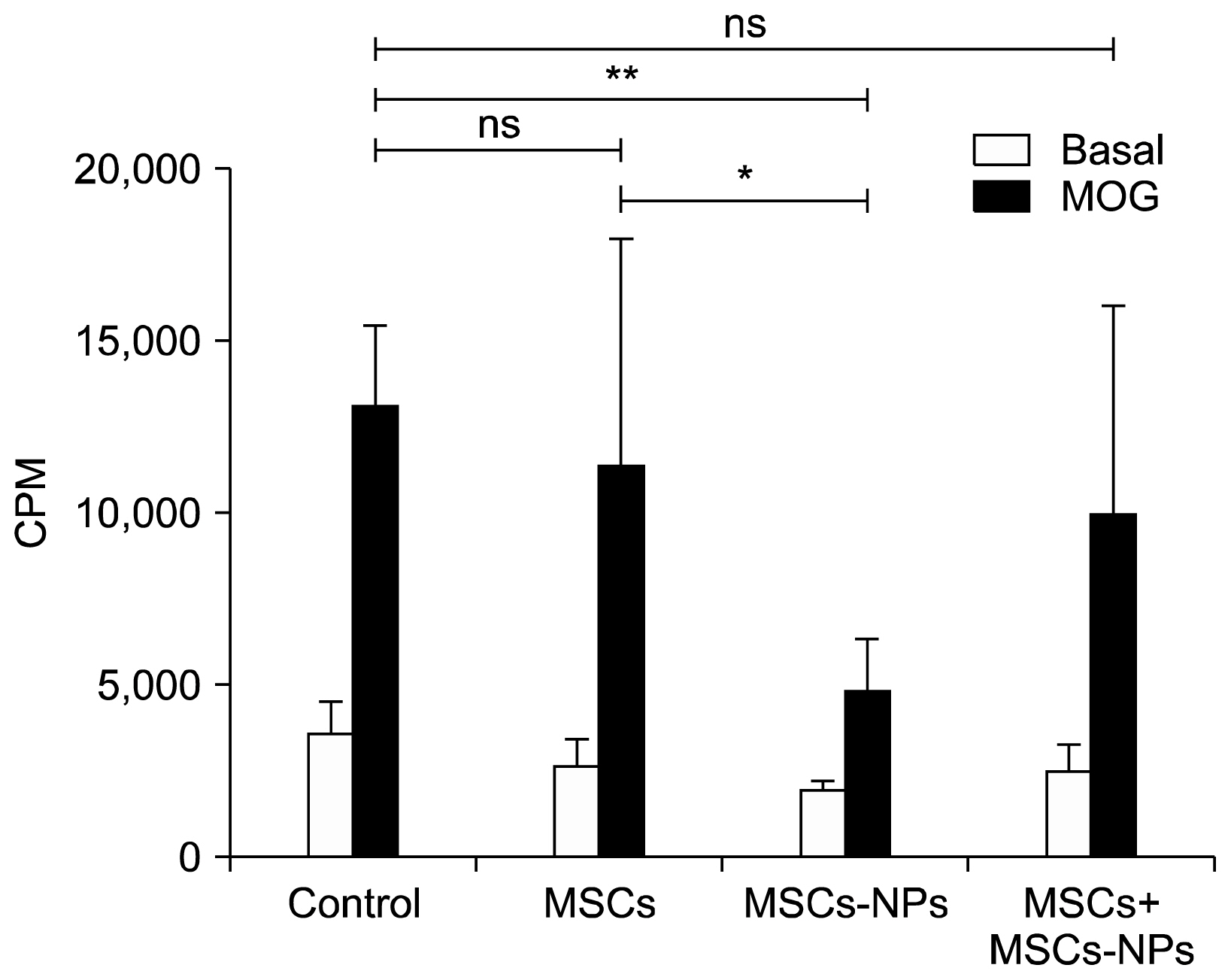
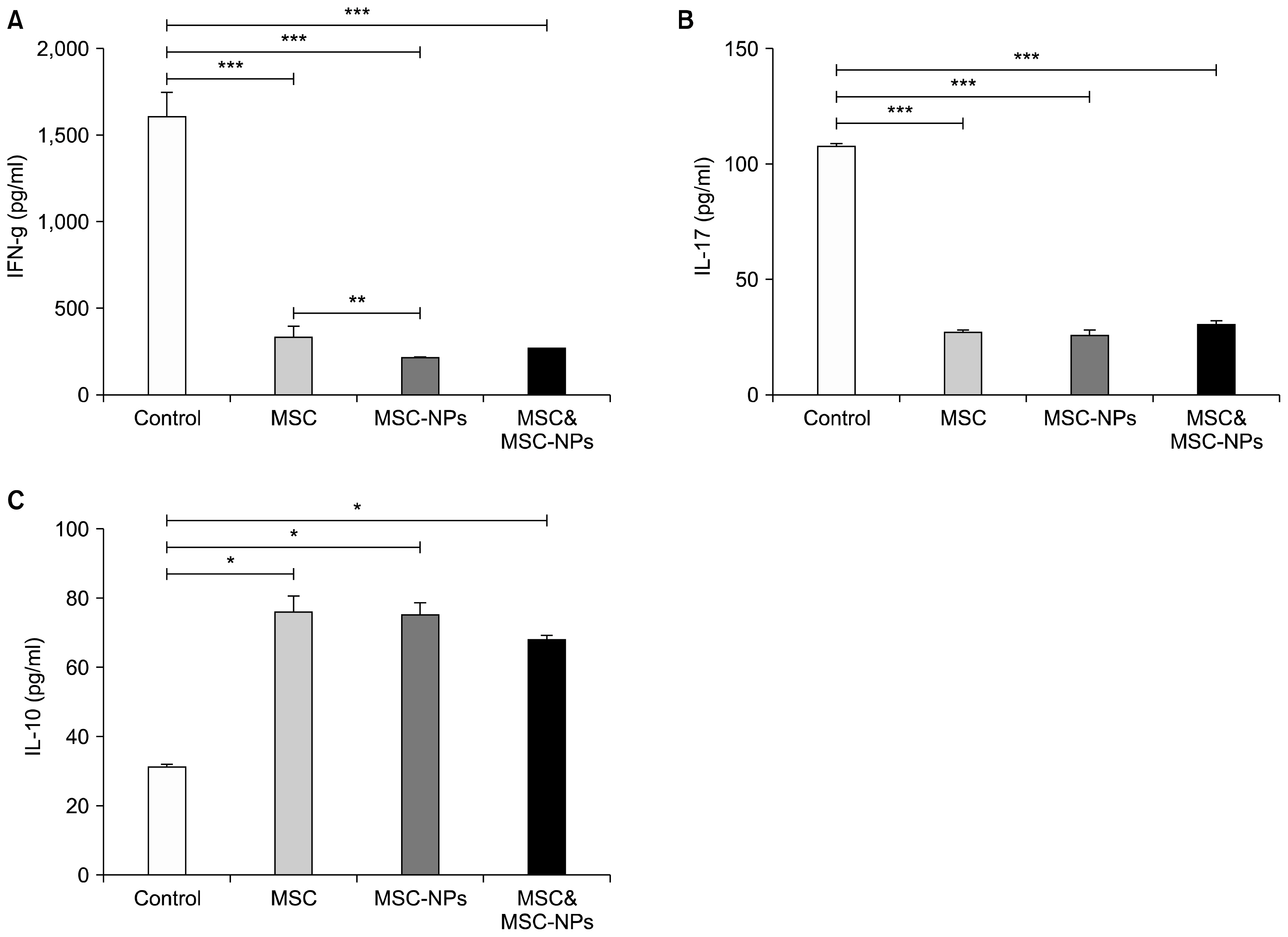
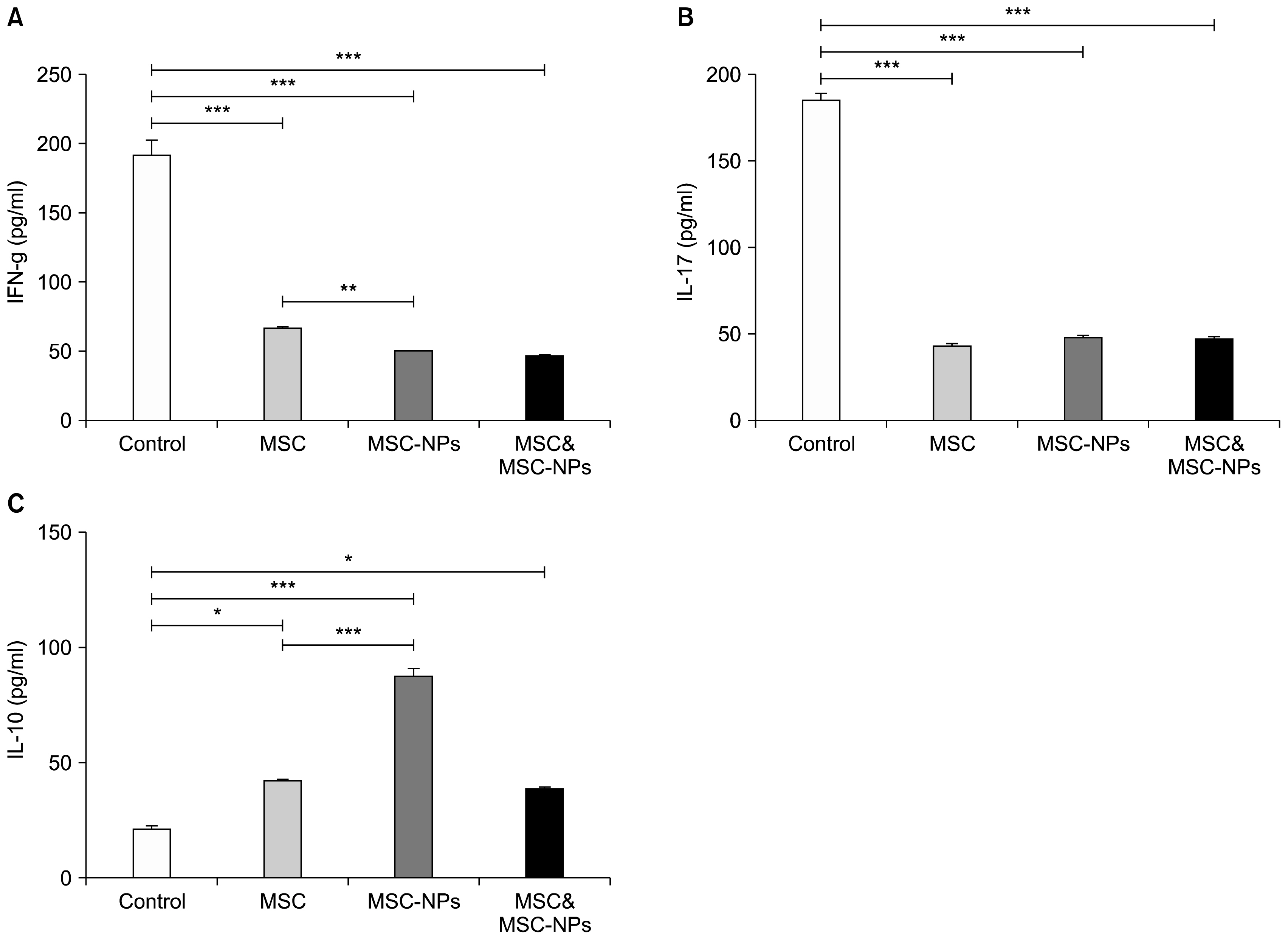
MSCs, MSCs-NPs and MSCs+MSCs-NP were administered intravenously to EAE mice on days 22, 29, and 36 post immunization. The levels of cytokines and PGE2 in sera or supernatant of in vitro cultured splenocytes derived from treated mice were measured by ELISA. The results of this study showed that in comparison to MSCs monotherapy, MSCs-NPs administration had a more profound capability of inhibiting the proliferation of pathogenic MOG35-55-specific T cells, decreasing IFN-γ production and increasing anti-inflammatory IL-10 cytokine production. These findings could be explained by higher ability of in vitro cultured MSCs-NPs in production of PGE2 compared to MSCs. In line with these findings, while the administration of MSCs and MSCs-NPs significantly decreased the clinical scores of EAE in comparison with the untreated EAE group, MSCs-NPs were significantly more efficient in reducing clinical score compared to MSCs. Of interest, combined therapy with MSCs and MSCs-NPs did not provide any benefit over monotherapy with MSCs-NPs.
CONCLUSIONS
In comparison to MSCs, allogenic MSCs-NPs are more potent in the attenuation of EAE.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Ace Card to Address Autoimmune Diseases
Hussein Baharlooi, Maryam Azimi, Zahra Salehi, Maryam Izad
Int J Stem Cells. 2019;13(1):13-23. doi: 10.15283/ijsc19108.rBMSCs/ITGA5B1 Promotes Human Vascular Smooth Muscle Cell Differentiation via Enhancing Nitric Oxide Production
Yingxin Zhang, Jie Ding, Cong Xu, Hongli Yang, Peng Xia, Shengjun Ma, Haiying Chen
Int J Stem Cells. 2018;11(2):168-176. doi: 10.15283/ijsc18079.
Reference
-
References
1. Park TY, Park SD, Cho JY, Moon JS, Kim NY, Park K, Seong RH, Lee SW, Morio T, Bothwell AL, Lee SK. ROR γt-specific transcriptional interactomic inhibition suppresses autoimmunity associated with TH17 cells. Proc Natl Acad Sci U S A. 2014; 111:18673–18678. DOI: 10.1073/pnas.1413687112. PMID: 25527718. PMCID: 4284575.
Article2. Yang C, He D, Yin C, Tan J. Inhibition of interferon regulatory factor 4 suppresses Th1 and Th17 cell differentiation and ameliorates experimental autoimmune encephalomyelitis. Scand J Immunol. 2015; 82:345–351. DOI: 10.1111/sji.12334. PMID: 26110284.
Article3. Grifka-Walk HM, Giles DA, Segal BM. IL-12-polarized Th1 cells produce GM-CSF and induce EAE independent of IL-23. Eur J Immunol. 2015; 45:2780–2786. DOI: 10.1002/eji.201545800. PMID: 26220255. PMCID: 5352159.
Article4. Yeh WI, McWilliams IL, Harrington LE. Autoreactive Tbet-positive CD4 T cells develop independent of classic Th1 cytokine signaling during experimental autoimmune encephalomyelitis. J Immunol. 2011; 187:4998–5006. DOI: 10.4049/jimmunol.1100031. PMID: 21984703. PMCID: 3709433.
Article5. Liu SP, Fu RH, Huang SJ, Huang YC, Chen SY, Chang CH, Liu CH, Tsai CH, Shyu WC, Lin SZ. Stem cell applications in regenerative medicine for neurological disorders. Cell Transplant. 2013; 22:631–637. DOI: 10.3727/096368912X655145.
Article6. Payne NL, Sun G, McDonald C, Moussa L, Emerson-Webber A, Loisel-Meyer S, Medin JA, Siatskas C, Bernard CC. Human adipose-derived mesenchymal stem cells engineered to secrete IL-10 inhibit APC function and limit CNS autoimmunity. Brain Behav Immun. 2013; 30:103–114. DOI: 10.1016/j.bbi.2013.01.079. PMID: 23369732.
Article7. Hermann A, Gastl R, Liebau S, Popa MO, Fiedler J, Boehm BO, Maisel M, Lerche H, Schwarz J, Brenner R, Storch A. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci. 2004; 117:4411–4422. DOI: 10.1242/jcs.01307. PMID: 15304527.
Article8. Kabos P, Ehtesham M, Kabosova A, Black KL, Yu JS. Generation of neural progenitor cells from whole adult bone marrow. Exp Neurol. 2002; 178:288–293. DOI: 10.1006/exnr.2002.8039. PMID: 12504887.
Article9. Constantin G, Marconi S, Rossi B, Angiari S, Calderan L, Anghileri E, Gini B, Bach SD, Martinello M, Bifari F, Galiè M, Turano E, Budui S, Sbarbati A, Krampera M, Bonetti B. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009; 27:2624–2635. DOI: 10.1002/stem.194. PMID: 19676124.
Article10. Zhu J, Zhang J, Li Q, Du Y, Qiao B, Hu X. Transplanting of mesenchymal stem cells may affect proliferation and function of CD4(+)T cells in experimental autoimmune encephalomyelitis. Exp Clin Transplant. 2012; 10:492–500. DOI: 10.6002/ect.2011.0197. PMID: 22817386.
Article11. Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, Miller SD, Miller RH. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009; 57:1192–1203. DOI: 10.1002/glia.20841. PMID: 19191336. PMCID: 2706928.
Article12. Rafei M, Birman E, Forner K, Galipeau J. Allogeneic mesenchymal stem cells for treatment of experimental autoimmune encephalomyelitis. Mol Ther. 2009; 17:1799–1803. DOI: 10.1038/mt.2009.157. PMID: 19602999. PMCID: 2835011.
Article13. Kassis I, Petrou P, Halimi M, Karussis D. Mesenchymal stem cells (MSC) derived from mice with experimental autoimmune encephalomyelitis (EAE) suppress EAE and have similar biological properties with MSC from healthy donors. Immunol Lett. 2013; 154:70–76. DOI: 10.1016/j.imlet.2013.06.002. PMID: 23994102.
Article14. Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005; 106:1755–1761. DOI: 10.1182/blood-2005-04-1496. PMID: 15905186.
Article15. Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003; 422:688–694. DOI: 10.1038/nature01552.
Article16. Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005; 436:266–271. DOI: 10.1038/nature03889. PMID: 16015332.
Article17. Yang J, Yan Y, Ciric B, Yu S, Guan Y, Xu H, Rostami A, Zhang GX. Evaluation of bone marrow- and brain-derived neural stem cells in therapy of central nervous system autoimmunity. Am J Pathol. 2010; 177:1989–2001. DOI: 10.2353/ajpath.2010.091203. PMID: 20724590. PMCID: 2947293.
Article18. Einstein O, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Polyzoidou E, Lavon I, Milonas I, Karussis D, Abramsky O, Ben-Hur T. Transplanted neural precursor cells reduce brain inflammation to attenuate chronic experimental autoimmune encephalomyelitis. Exp Neurol. 2006; 198:275–284. DOI: 10.1016/j.expneurol.2005.11.007. PMID: 16472805.
Article19. Harris VK, Yan QJ, Vyshkina T, Sahabi S, Liu X, Sadiq SA. Clinical and pathological effects of intrathecal injection of mesenchymal stem cell-derived neural progenitors in an experimental model of multiple sclerosis. J Neurol Sci. 2012; 313:167–177. DOI: 10.1016/j.jns.2011.08.036.
Article20. Shiri EH, Mehrjardi NZ, Tavallaei M, Ashtiani SK, Baharvand H. Neurogenic and mitotic effects of dehydroepiandrosterone on neuronal-competent marrow mesenchymal stem cells. Int J Dev Biol. 2009; 53:579–584. DOI: 10.1387/ijdb.082623eh. PMID: 19378256.
Article21. Grigoriadis N, Lourbopoulos A, Lagoudaki R, Frischer JM, Polyzoidou E, Touloumi O, Simeonidou C, Deretzi G, Kountouras J, Spandou E, Kotta K, Karkavelas G, Tascos N, Lassmann H. Variable behavior and complications of autologous bone marrow mesenchymal stem cells transplanted in experimental autoimmune encephalomyelitis. Exp Neurol. 2011; 230:78–89. DOI: 10.1016/j.expneurol.2011.02.021. PMID: 21440544.
Article22. Harris VK, Faroqui R, Vyshkina T, Sadiq SA. Characterization of autologous mesenchymal stem cell-derived neural progenitors as a feasible source of stem cells for central nervous system applications in multiple sclerosis. Stem Cells Transl Med. 2012; 1:536–547. DOI: 10.5966/sctm.2012-0015. PMID: 23197858. PMCID: 3659719.
Article23. Hsu WT, Lin CH, Chiang BL, Jui HY, Wu KK, Lee CM. Prostaglandin E2 potentiates mesenchymal stem cell-induced IL-10+IFN-γ+CD4+ regulatory T cells to control transplant arteriosclerosis. J Immunol. 2013; 190:2372–2380. DOI: 10.4049/jimmunol.1202996. PMID: 23359497.
Article24. Duffy MM, Pindjakova J, Hanley SA, McCarthy C, Weidhofer GA, Sweeney EM, English K, Shaw G, Murphy JM, Barry FP, Mahon BP, Belton O, Ceredig R, Griffin MD. Mesenchymal stem cell inhibition of T-helper 17 cell-differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol. 2011; 41:2840–2851. DOI: 10.1002/eji.201141499. PMID: 21710489.
Article25. Wang L, Shi J, van Ginkel FW, Lan L, Niemeyer G, Martin DR, Snyder EY, Cox NR. Neural stem/progenitor cells modulate immune responses by suppressing T lymphocytes with nitric oxide and prostaglandin E2. Exp Neurol. 2009; 216:177–183. DOI: 10.1016/j.expneurol.2008.11.017.
Article26. Matysiak M, Orlowski W, Fortak-Michalska M, Jurewicz A, Selmaj K. Immunoregulatory function of bone marrow mesenchymal stem cells in EAE depends on their differentiation state and secretion of PGE2. J Neuroimmunol. 2011; 233:106–111. DOI: 10.1016/j.jneuroim.2010.12.004. PMID: 21354631.
Article27. Yang J, Jiang Z, Fitzgerald DC, Ma C, Yu S, Li H, Zhao Z, Li Y, Ciric B, Curtis M, Rostami A, Zhang GX. Adult neural stem cells expressing IL-10 confer potent immunomodulation and remyelination in experimental autoimmune encephalitis. J Clin Invest. 2009; 119:3678–3691. DOI: 10.1172/JCI37914. PMID: 19884657. PMCID: 2786785.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Ace Card to Address Autoimmune Diseases
- Current Trends and Prospect of Cell Therapy using Hematopoietic Stem Cells
- Preclinical Efficacy and Mechanisms of Mesenchymal Stem Cells in Animal Models of Autoimmune Diseases
- Recent Trends and Strategies in Stem Cell Therapy for Alzheimer's Disease