J Vet Sci.
2017 Mar;18(1):59-65. 10.4142/jvs.2017.18.1.59.
Inhibition by miR-410 facilitates direct retinal pigment epithelium differentiation of umbilical cord blood-derived mesenchymal stem cells
- Affiliations
-
- 1Adult Stem Cell Research Center, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea. kangpub@snu.ac.kr
- 2Research Institute for Veterinary Science, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea.
- KMID: 2412588
- DOI: http://doi.org/10.4142/jvs.2017.18.1.59
Abstract
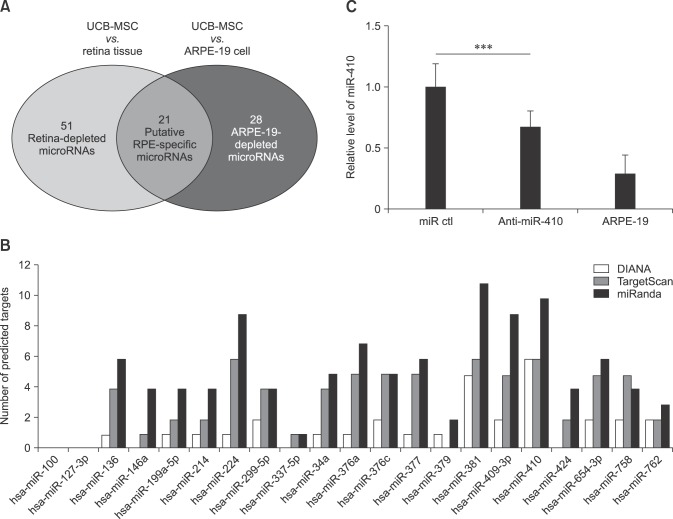
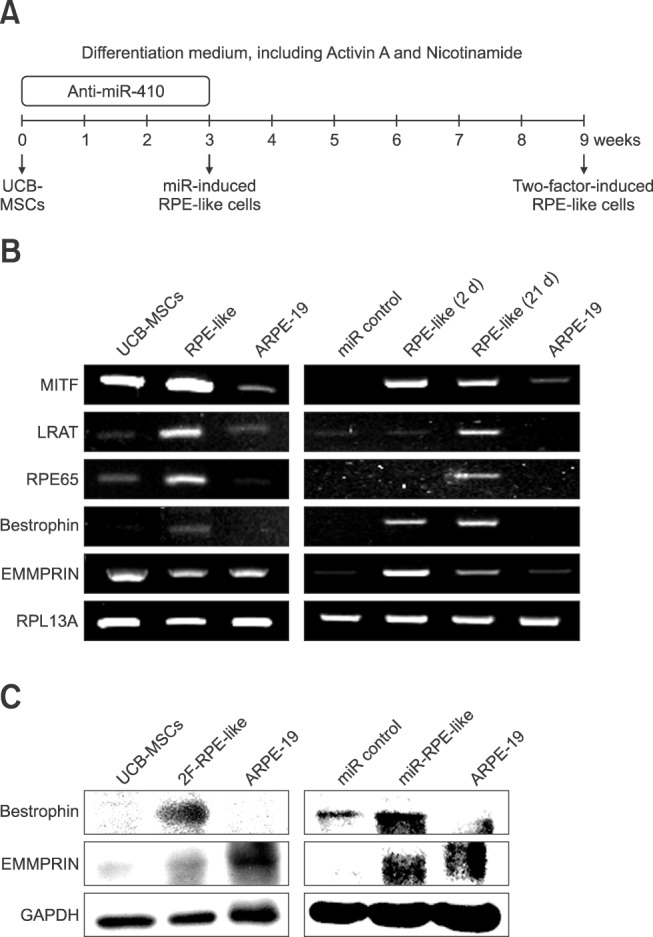
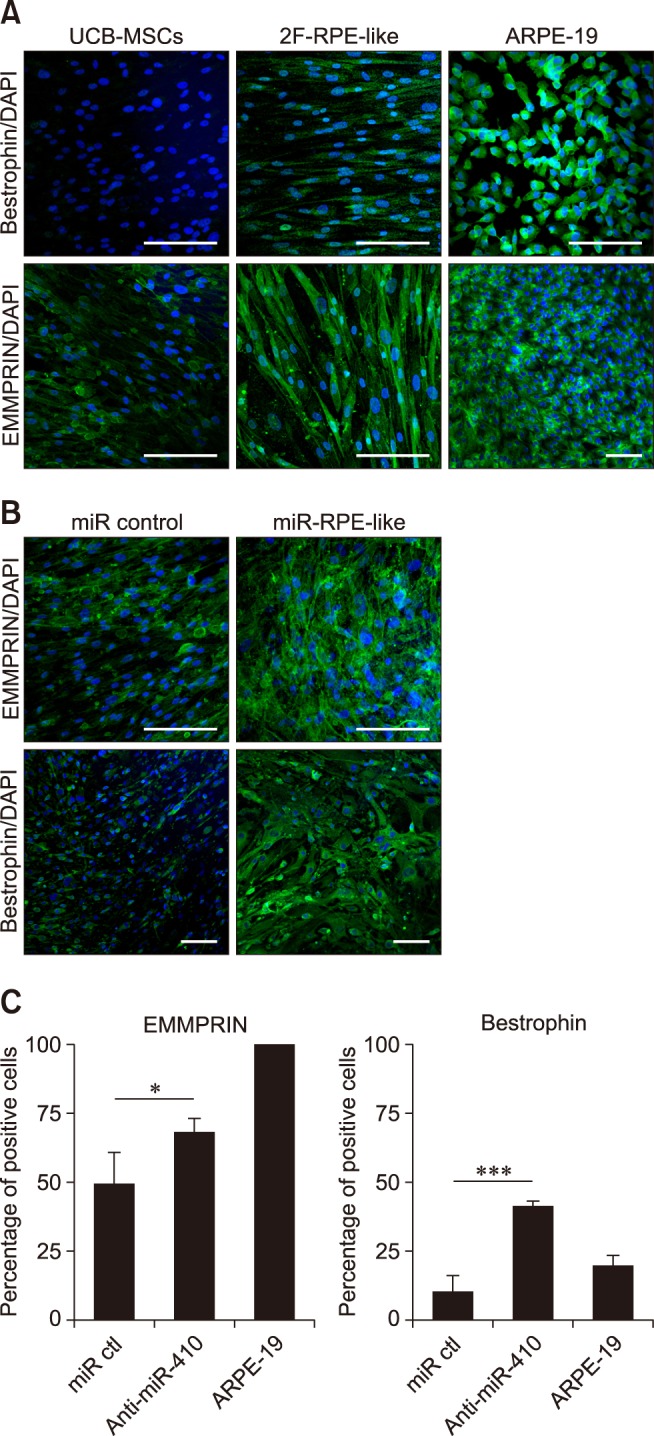
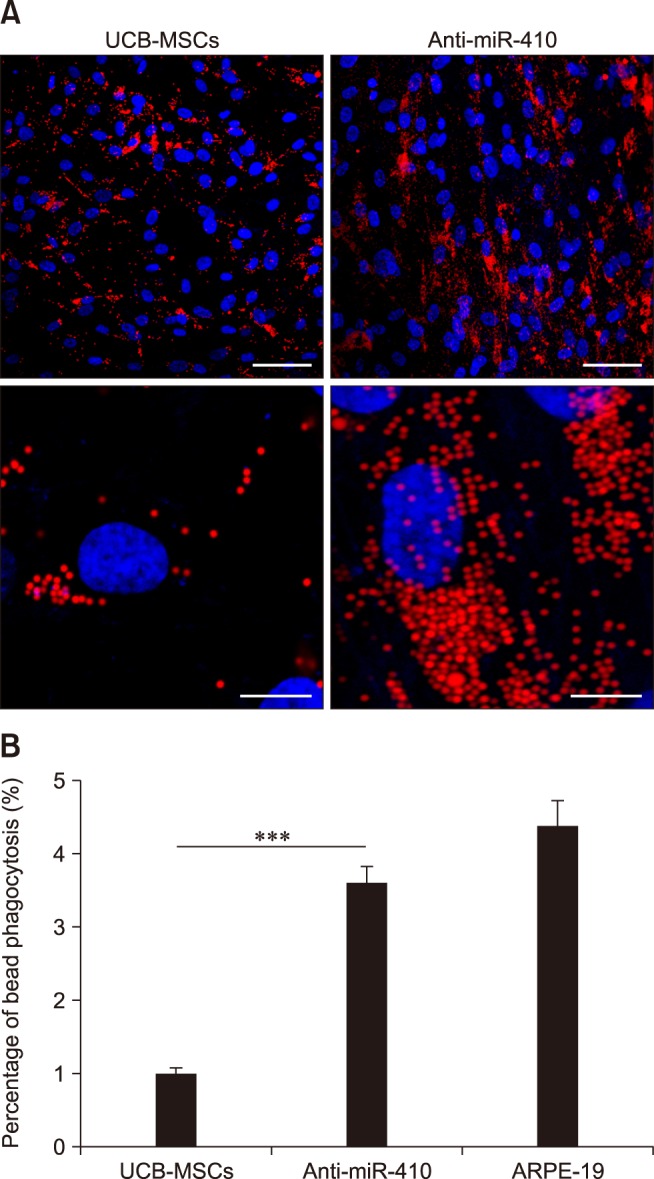
- Retinal pigment epithelium (RPE) is a major component of the eye. This highly specialized cell type facilitates maintenance of the visual system. Because RPE loss induces an irreversible visual impairment, RPE generation techniques have recently been investigated as a potential therapeutic approach to RPE degeneration. The microRNA-based technique is a new strategy for producing RPE cells from adult stem cell sources. Previously, we identified that antisense microRNA-410 (anti-miR-410) induces RPE differentiation from amniotic epithelial stem cells. In this study, we investigated RPE differentiation from umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs) via anti-miR-410 treatment. We identified miR-410 as a RPE-relevant microRNA in UCB-MSCs from among 21 putative human RPE-depleted microRNAs. Inhibition of miR-410 induces overexpression of immature and mature RPE-specific factors, including MITF, LRAT, RPE65, Bestrophin, and EMMPRIN. The RPE-induced cells were able to phagocytize microbeads. Results of our microRNA-based strategy demonstrated proof-of-principle for RPE differentiation in UCB-MSCs by using anti-miR-410 treatment without the use of additional factors or exogenous transduction.
Keyword
MeSH Terms
-
Cell Differentiation/*genetics
Fetal Blood/cytology/metabolism
Gene Expression Regulation, Developmental
Humans
Mesenchymal Stromal Cells/cytology/metabolism
MicroRNAs/genetics/*metabolism
Otx Transcription Factors/*biosynthesis
Phagocytosis
Retinal Pigment Epithelium/metabolism/*physiology
cis-trans-Isomerases/*biosynthesis
MicroRNAs
Otx Transcription Factors
cis-trans-Isomerases
Figure
Reference
-
1. Aoki H, Hara A, Nakagawa S, Motohashi T, Hirano M, Takahashi Y, Kunisada T. Embryonic stem cells that differentiate into RPE cell precursors in vitro develop into RPE cell monolayers in vivo. Exp Eye Res. 2006; 82:265–274. PMID: 16150443.
Article2. Buchholz DE, Hikita ST, Rowland TJ, Friedrich AM, Hinman CR, Johnson LV, Clegg DO. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009; 27:2427–2434. PMID: 19658190.
Article3. Chen L, Zhang J, Feng Y, Li R, Sun X, Du W, Piao X, Wang H, Yang D, Sun Y, Li X, Jiang T, Kang C, Li Y, Jiang C. MiR-410 regulates MET to influence the proliferation and invasion of glioma. Int J Biochem Cell Biol. 2012; 44:1711–1717. PMID: 22750473.
Article4. Chien WW, Domenech C, Catallo R, Kaddar T, Magaud JP, Salles G, Ffrench M. Cyclin-dependent kinase 1 expression is inhibited by p16INK4a at the post-transcriptional level through the microRNA pathway. Oncogene. 2011; 30:1880–1891. PMID: 21170085.5. Choi SW, Kim JJ, Seo MS, Park SB, Kang TW, Lee JY, Lee BC, Kang I, Shin TH, Kim HS, Yu KR, Kang KS. miR-410 inhibition induces RPE differentiation of amniotic epithelial stem cells via overexpression of OTX2 and RPE65. Stem Cell Rev. 2015; 11:376–386. PMID: 25351180.
Article6. Diep DB, Hoen N, Backman M, Machon O, Krauss S. Characterisation of the Wnt antagonists and their response to conditionally activated Wnt signalling in the developing mouse forebrain. Brain Res Dev Brain Res. 2004; 153:261–270. PMID: 15527894.
Article7. Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996; 62:155–169. PMID: 8698076.
Article8. Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998; 391:357–362. PMID: 9450748.
Article9. Idelson M, Alper R, Obolensky A, Ben-Shushan E, Hemo I, Yachimovich-Cohen N, Khaner H, Smith Y, Wiser O, Gropp M, Cohen MA, Even-Ram S, Berman-Zaken Y, Matzrafi L, Rechavi G, Banin E, Reubinoff B. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009; 5:396–408. PMID: 19796620.
Article10. Kokkinaki M, Sahibzada N, Golestaneh N. Human induced pluripotent stem-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized vascular endothelial growth factor secretion, and gene expression pattern similar to native RPE. Stem Cells. 2011; 29:825–835. PMID: 21480547.
Article11. Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopolous GD, Harland RM. Neural induction by the secreted polypeptide noggin. Science. 1993; 262:713–718. PMID: 8235591.
Article12. Liao JL, Yu J, Huang K, Hu J, Diemer T, Ma Z, Dvash T, Yang XJ, Travis GH, Williams DS, Bok D, Fan G. Molecular signature of primary retinal pigment epithelium and stem-cell-derived RPE cells. Hum Mol Genet. 2010; 19:4229–4238. PMID: 20709808.
Article13. Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, Akaike A, Sasai Y, Takahashi M. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008; 26:215–224. PMID: 18246062.
Article14. Osakada F, Ikeda H, Sasai Y, Takahashi M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protoc. 2009; 4:811–824. PMID: 19444239.
Article15. Park SB, Yu KR, Jung JW, Lee SR, Roh KH, Seo MS, Park JR, Kang SK, Lee YS, Kang KS. bFGF enhances the IGFs-mediated pluripotent and differentiation potentials in multipotent stem cells. Growth Factors. 2009; 27:425–437. PMID: 19919531.
Article16. Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012; 96:614–618. PMID: 22133988.
Article17. Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004; 82:844–851. PMID: 15640920.18. Roh DH, Seo MS, Choi HS, Park SB, Han HJ, Beitz AJ, Kang KS, Lee JH. Transplantation of human umbilical cord blood or amniotic epithelial stem cells alleviates mechanical allodynia after spinal cord injury in rats. Cell Transplant. 2013; 22:1577–1590. PMID: 23294734.
Article19. Snyder CM, Rice AL, Estrella NL, Held A, Kandarian SC, Naya FJ. MEF2A regulates the Gtl2-Dio3 microRNA mega-cluster to modulate WNT signaling in skeletal muscle regeneration. Development. 2013; 140:31–42. PMID: 23154418.20. Sonoda S, Spee C, Barron E, Ryan SJ, Kannan R, Hinton DR. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat Protoc. 2009; 4:662–673. PMID: 19373231.
Article21. Tang C, Lee AS, Volkmer JP, Sahoo D, Nag D, Mosley AR, Inlay MA, Ardehali R, Chavez SL, Pera RR, Behr B, Wu JC, Weissman IL, Drukker M. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011; 29:829–834. PMID: 21841799.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Difference in HLA-DR Expression of Human Umbilical Cord Blood Derived Mesenchymal Stem Cells after Tri-lineage Differentiation
- Difference in Cell Characteristics among the Monoclonal Cell Populations Obtained from the Human Umbilical Cord Blood Derived Mesenchymal Stem Cell Population
- A Simple Method to Isolate and Expand Human Umbilical Cord Derived Mesenchymal Stem Cells: Using Explant Method and Umbilical Cord Blood Serum