J Vet Sci.
2017 Mar;18(1):33-38. 10.4142/jvs.2017.18.1.33.
A serological study of severe fever with thrombocytopenia syndrome using a virus neutralization test and competitive enzyme-linked immunosorbent assay
- Affiliations
-
- 1Viral Disease Division, Animal and Plant Quarantine Agency, Gimcheon 39660, Korea. shinyk2009@korea.kr
- 2Foreign Animal Disease Division, Animal and Plant Quarantine Agency, Gimcheon 39660, Korea.
- KMID: 2412585
- DOI: http://doi.org/10.4142/jvs.2017.18.1.33
Abstract
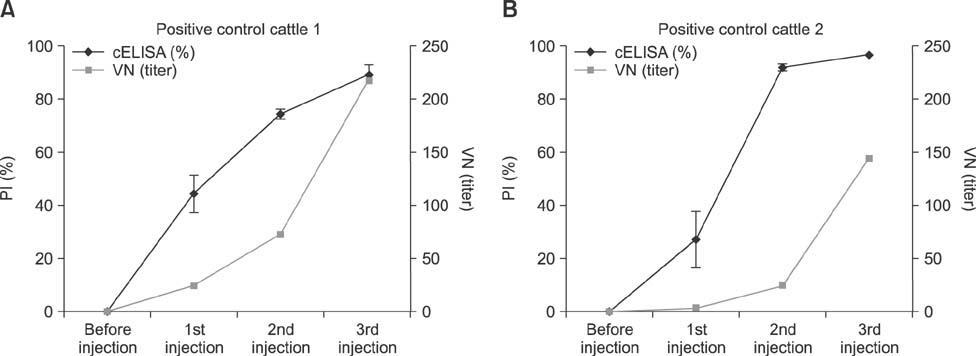
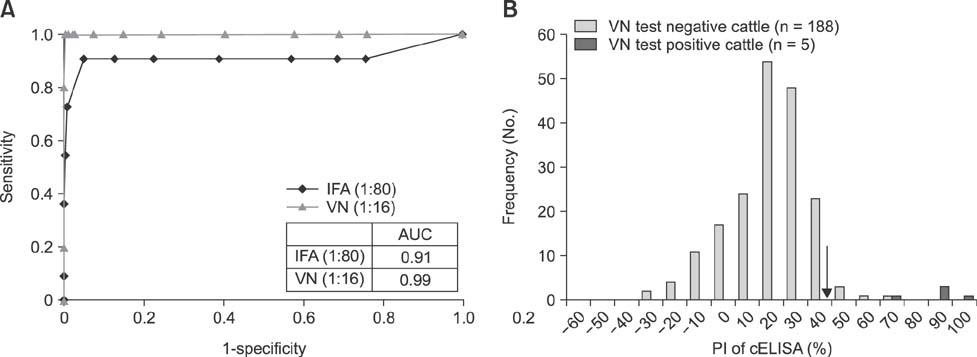
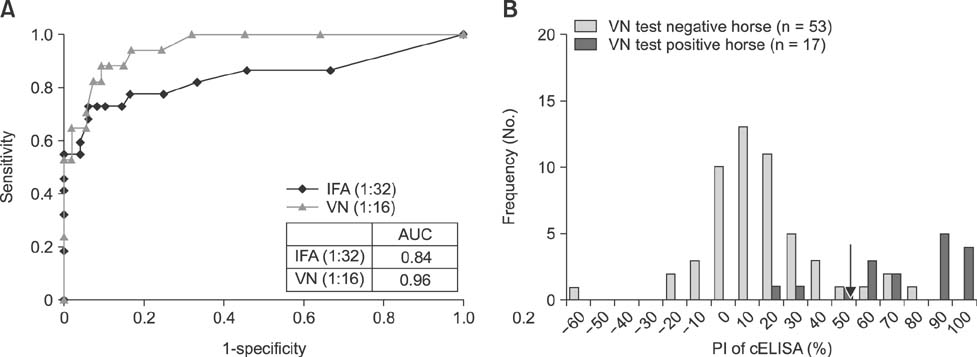
- Severe fever with thrombocytopenia syndrome (SFTS) is caused by the SFTS virus (SFTSV). The SFTSV appears to have a wide host range, as SFTSV-positive ticks have been isolated from both farm animals and wild rodents. Therefore, it is important to monitor SFTSV-positive animals to prevent the transmission of SFTSV from animals to humans. Previously, we developed a competitive enzyme-linked immunosorbent assay (cELISA) to detect SFTSV-specific antibodies from field animals and compared the cELISA results to those from an indirect immunofluorescence assay (IFA). In this study, cELISA results were compared to and evaluated against the results from both an IFA and a virus neutralization (VN) test of 193 bovine serum samples (including two bovine positive control sera) and 70 horse serum samples. The consistency (98.9%) between cELISA and VN results was higher than that (97.4%) between cELISA and IFA for the bovine serum samples. Similarly, for the horse serum samples, the consistency (88.6%) between cELISA and VN results was higher than that (84.3%) between the cELISA and IFA. These findings indicate that our newly developed cELISA can be used for surveillance or epidemiological studies of SFTSV in animals.
Keyword
MeSH Terms
-
Animals
Cattle
Cattle Diseases/*diagnosis/virology
Enzyme-Linked Immunosorbent Assay/*veterinary
Fluorescent Antibody Technique, Indirect/*veterinary
Horse Diseases/*diagnosis/virology
Horses
Neutralization Tests/*veterinary
Phlebotomus Fever/diagnosis/*veterinary/virology
Phlebovirus/*isolation & purification
Thrombocytopenia/diagnosis/veterinary/virology
Figure
Reference
-
1. Ammerman NC, Beier-Sexton M, Azad AF. Growth and maintenance of Vero cell lines. Curr Protoc Microbiol. 2008; Appendix 4:Appendix 4E.
Article2. Bosco-Lauth AM, Panella NA, Root JJ, Gidlewski T, Lash RR, Harmon JR, Burkhalter KL, Godsey MS, Savage HM, Nicholson WL, Komar N, Brault AC. Serological investigation of heartland virus (Bunyaviridae: Phlebovirus) exposure in wild and domestic animals adjacent to human case sites in Missouri 2012-2013. Am J Trop Med Hyg. 2015; 92:1163–1167.
Article3. Cliquet F, Aubert M, Sagné L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J Immunol Methods. 1998; 212:79–87.
Article4. Doan HT, Noh JH, Choe SE, Yoo MS, Kim YH, Reddy KE, Quyen DV, Nguyen LT, Nguyen TT, Kweon CH, Jung SC, Chang KY, Kang SW. Molecular detection and phylogenetic analysis of Anaplasma bovis from Haemaphysalis longicornis feeding on grazing cattle in Korea. Vet Parasitol. 2013; 196:478–481.
Article5. Guo X, Zhang L, Zhang W, Chi Y, Zeng X, Li X, Qi X, Jin Q, Zhang X, Huang M, Wang H, Chen Y, Bao C, Hu J, Liang S, Bao L, Wu T, Zhou M, Jiao Y. Human antibody neutralizes severe fever with thrombocytopenia syndrome virus, an emerging hemorrhagic fever virus. Clin Vaccine Immunol. 2013; 20:1426–1432.
Article6. Jiao Y, Zeng X, Guo X, Qi X, Zhang X, Shi Z, Zhou M, Bao C, Zhang W, Xu Y, Wang H. Preparation and evaluation of recombinant severe fever with thrombocytopenia syndrome virus nucleocapsid protein for detection of total antibodies in human and animal sera by double-antigen sandwich enzyme-linked immunosorbent assay. J Clin Microbiol. 2012; 50:372–377.
Article7. Kim CM, Kim MS, Park MS, Park JH, Chae JS. Identification of Ehrlichia chaffeensis, Anaplasma phagocytophilum, and A. bovis in Haemaphysalis longicornis and Ixodes persulcatus ticks from Korea. Vector Borne Zoonotic Dis. 2003; 3:17–26.8. Kim KH, Yi J, Kim G, Choi SJ, Jun KI, Kim NH, Choe PG, Kim NJ, Lee JK, Oh MD. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis. 2013; 19:1892–1894.
Article9. Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977; 33:363–374.
Article10. Lee H, Kim EJ, Song JY, Choi JS, Lee JY, Cho IS, Shin YK. Development and evaluation of a competitive enzyme-linked immunosorbent assay using a monoclonal antibody for the diagnosis of severe fever with thrombocytopenia syndrome virus in bovine sera. J Vet Sci. 2016; 17:307–314.
Article11. Lei XY, Liu MM, Yu XJ. Severe fever with thrombocytopenia syndrome and its pathogen SFTSV. Microbes Infect. 2015; 17:149–154.
Article12. Libeau G, Diallo A, Calvez D, Lefèvre PC. A competitive ELISA using anti-N monoclonal antibodies for specific detection of rinderpest antibodies in cattle and small ruminants. Vet Microbiol. 1992; 31:147–160.
Article13. McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, Metcalfe MG, Batten BC, Albariño CG, Zaki SR, Rollin PE, Nicholson WL, Nichol ST. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012; 367:834–841.
Article14. Niu G, Li J, Liang M, Jiang X, Jiang M, Yin H, Wang Z, Li C, Zhang Q, Jin C, Wang X, Ding S, Xing Z, Wang S, Bi Z, Li D. Severe fever with thrombocytopenia syndrome virus among domesticated animals, China. Emerg Infect Dis. 2013; 19:756–763.
Article15. Park SW, Song BG, Shin EH, Yun SM, Han MG, Park MY, Park C, Ryou J. Prevalence of severe fever with thrombocytopenia syndrome virus in Haemaphysalis longicornis ticks in South Korea. Ticks Tick Borne Dis. 2014; 5:975–977.
Article16. Swei A, Russell BJ, Naccache SN, Kabre B, Veeraraghavan N, Pilgard MA, Johnson BJ, Chiu CY. The genome sequence of Lone Star virus, a highly divergent bunyavirus found in the Amblyomma americanum tick. PLoS One. 2013; 8:e62083.
Article17. Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, Kamei T, Honda M, Ninomiya D, Sakai T, Senba T, Kaneyuki S, Sakaguchi S, Satoh A, Hosokawa T, Kawabe Y, Kurihara S, Izumikawa K, Kohno S, Azuma T, Suemori K, Yasukawa M, Mizutani T, Omatsu T, Katayama Y, Miyahara M, Ijuin M, Doi K, Okuda M, Umeki K, Saito T, Fukushima K, Nakajima K, Yoshikawa T, Tani H, Fukushi S, Fukuma A, Ogata M, Shimojima M, Nakajima N, Nagata N, Katano H, Fukumoto H, Sato Y, Hasegawa H, Yamagishi T, Oishi K, Kurane I, Morikawa S, Saijo M. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J Infect Dis. 2014; 209:816–827.
Article18. Yu L, Zhang L, Sun L, Lu J, Wu W, Li C, Zhang Q, Zhang F, Jin C, Wang X, Bi Z, Li D, Liang M. Critical epitopes in the nucleocapsid protein of SFTS virus recognized by a panel of SFTS patients derived human monoclonal antibodies. PLoS One. 2012; 7:e38291.
Article19. Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011; 364:1523–1532.
Article20. Yun SM, Lee WG, Ryou J, Yang SC, Park SW, Roh JY, Lee YJ, Park C, Han MG. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg Infect Dis. 2014; 20:1358–1361.
Article21. Zhao L, Zhai S, Wen H, Cui F, Chi Y, Wang L, Xue F, Wang Q, Wang Z, Zhang S, Song Y, Du J, Yu XJ. Severe fever with thrombocytopenia syndrome virus, Shandong Province, China. Emerg Infect Dis. 2012; 18:963–965.
Article22. Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993; 39:561–577.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of two different enzyme-linked immunosorbent assay for severe fever with thrombocytopenia syndrome virus diagnosis
- Development and evaluation of a competitive enzyme-linked immunosorbent assay using a monoclonal antibody for diagnosis of severe fever with thrombocytopenia syndrome virus in bovine sera
- Correlations in the results of virus neutralization test, hemagglutination inhibition test, and enzyme-linked immunosorbent assay to determine infectious bronchitis virus vaccine potency
- Two Cases of Dengue Fever Due to Dengue Virus-1 Developed in a Family
- Generation, characterization, and application in serodiagnosis of recombinant swine vesicular disease virus-like particles