J Vet Sci.
2017 Jun;18(2):245-251. 10.4142/jvs.2017.18.2.245.
Efficacy of horse chestnut leaf extract ALH-L1005 as a matrix metalloproteinase inhibitor in ligature-induced periodontitis in canine model
- Affiliations
-
- 1Department of Veterinary Surgery, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea. kmseo@snu.ac.kr
- 2AngioLab, Inc. Daejeon 34016, Korea.
- KMID: 2412578
- DOI: http://doi.org/10.4142/jvs.2017.18.2.245
Abstract
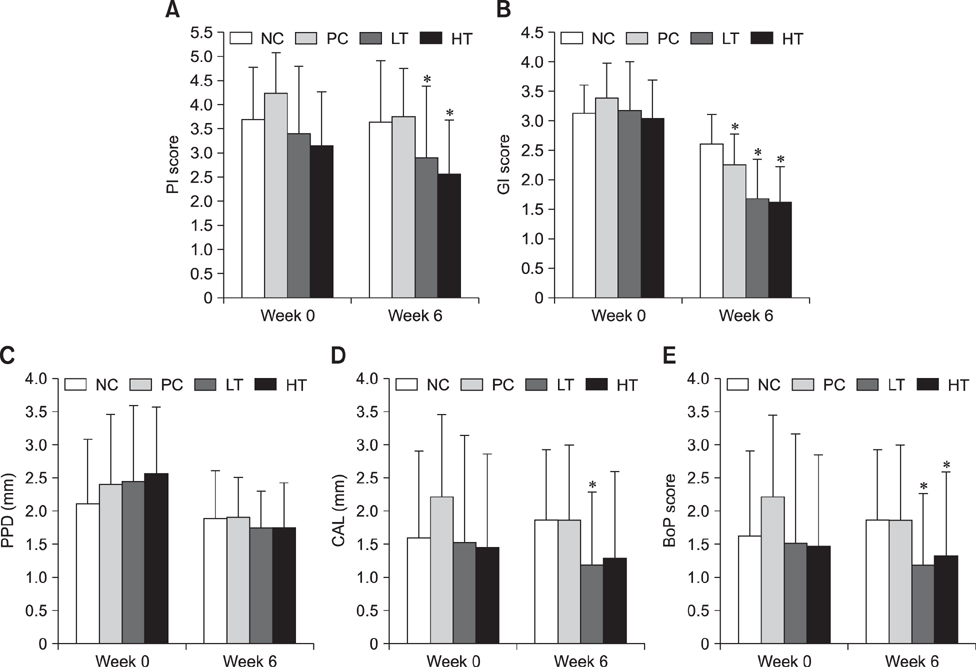
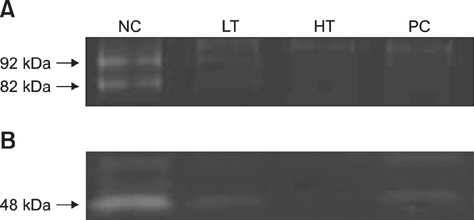
- Matrix metalloproteinases (MMPs) are the main proteinases associated with periodontal tissue destruction and remodeling. Therefore, inhibition of host-derived MMPs has a key role in the prevention and reduction of periodontitis progression. Horse chestnut (Aesculus hippocastanum L.) extracts have been used as treatments for inflammatory disease, traditionally. This study assessed the clinical effect as a MMP inhibitor of horse chestnut leaf extract ALH-L1005 on periodontitis. ALH-L1005 was obtained from horse chestnut leaf and its MMP inhibitory activities estimated. Periodontitis was induced in beagles assigned to 4 groups and medicated for 6 weeks: low dose test (LT; ALH-L1005, 100 mg/kg/day), high dose test (HT; ALH-L1005, 200 mg/kg/day), positive control (PC; doxycycline, 10 mg/kg/day), or negative control (NC; placebo). Before and after administration, clinical indices of the teeth and MMP quantity in gingival tissues using zymography were measured. Clinical conditions of the LT, HT, and PC groups were significantly improved after 6 weeks. In zymographic evaluations, gelatinolytic and caseinolytic activities were suppressed in LT, HT, and PC groups but not in the NC group. The results suggest that ALH-L1005 could be an effective agent for clinical prevention and treatment of periodontitis by inhibiting the gelatinase and collagenase activities, which can detach periodontal ligaments from alveolar bone.
Keyword
MeSH Terms
-
*Aesculus/chemistry
Animals
Disease Models, Animal
Dog Diseases/*drug therapy
Dogs
Dose-Response Relationship, Drug
Gingiva/metabolism/surgery
Ligation/adverse effects/veterinary
Matrix Metalloproteinase Inhibitors/*therapeutic use
Periodontitis/drug therapy/*veterinary
Plant Extracts/administration & dosage/*therapeutic use
*Plant Leaves/chemistry
Matrix Metalloproteinase Inhibitors
Plant Extracts
Figure
Reference
-
1. Bellows J. Periodontal equipment, materials, and techniques. Small Animal Dental Equipment, Materials and Techniques. A Primer. Ames: Blackwell;2004. p. 115–173.2. Bisset NG. Herbal Drugs and Phytopharmaceuticals. A Handbook for Practice on a Scientific Basis. Stuttgart: Medpharm Scientific;2001. p. 271–272.3. Chang B, Lee Y, Ku Y, Bae K, Chung C. Antimicrobial activity of magnolol and honokiol against periodontopathic microorganisms. Planta Med. 1998; 64:367–369.
Article4. Chen JM, Chen WT. Fibronectin-degrading proteases from the membranes of transformed cells. Cell. 1987; 48:193–203.
Article5. Choi DH, Moon IS, Choi BK, Paik JW, Kim YS, Choi SH, Kim CK. Effects of sub-antimicrobial dose doxycycline therapy on crevicular fluid MMP-8, and gingival tissue MMP-9, TIMP-1 and IL-6 levels in chronic periodontitis. J Periodontal Res. 2004; 39:20–26.
Article6. Duke JA. Handbook of Medicinal Herbs. Boca Raton: CRC Press;2001. p. 20.7. Escartin Q, Lallam-Laroye C, Baroukh B, Morvan FO, Caruelle JP, Godeau G, Barritault D, Saffar JL. A new approach to treat tissue destruction in periodontitis with chemically modified dextran polymers. FASEB J. 2003; 17:644–651.
Article8. Genco RJ. Host responses in periodontal diseases: current concepts. J Periodontol. 1992; 63:4 Suppl. 338–355.
Article9. Gürkan A, Cinarcik S, Hüseyinov A. Adjunctive subantimicrobial dose doxycycline: effect on clinical parameters and gingival crevicular fluid transforming growth factor-β1 levels in severe, generalized chronic periodontitis. J Clin Periodontol. 2005; 32:244–253.
Article10. Hernandez M, Valenzuela MA, Lopez-Otin C, Alvarez J, Lopez JM, Vernal R, Gamonal J. Matrix metalloproteinase-13 is highly expressed in destructive periodontal disease activity. J Periodontol. 2006; 77:1863–1870.
Article11. Hill PA, Docherty AJ, Bottomley KMK, O'Connell JP, Morphy JR, Reynolds JJ, Meikle MC. Inhibition of bone resorption in vitro by selective inhibitors of gelatinase and collagenase. Biochem J. 1995; 308:167–175.
Article12. Kinane DF. Regulators of tissue destruction and homeostasis as diagnostic aids in periodontology. Periodontol 2000. 2000; 24:215–225.
Article13. Knäuper V, Smith B, López-Otin C, Murphy G. Activation of progelatinase B (proMMP-9) by active collagenase-3 (MMP-13). Eur J Biochem. 1997; 248:369–373.
Article14. Kut-Lasserre C, Miller CC, Ejeil AL, Gogly B, Dridi M, Piccardi N, Guillou B, Pellat B, Godeau G. Effect of avocado and soybean unsaponifiables on gelatinase A (MMP-2), stromelysin 1 (MMP-3), and tissue inhibitors of matrix metalloproteinase (TIMP-1 and TIMP-2) secretion by human fibroblasts in culture. J Periodontol. 2001; 72:1685–1694.
Article15. Lana SE, Ogilvie GK, Hansen RA, Powers BE, Dernell WS, Withrow SJ. Identification of matrix metalloproteinases in canine neoplastic tissue. Am J Vet Res. 2000; 61:111–114.
Article16. Lee HM, Ciancio SG, Tüter G, Ryan ME, Komaroff E, Golub LM. Subantimicrobial dose doxycycline efficacy as a matrix metalloproteinase inhibitor in chronic periodontitis patients is enhanced when combined with a non-steroidal anti-inflammatory drug. J Periodontol. 2004; 75:453–463.
Article17. Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963; 21:533–551.
Article18. Mäkelä M, Salo T, Uitto VJ, Larjava H. Matrix metalloproteinases (MMP-2 and MMP-9) of the oral cavity: cellular origin and relationship to periodontal status. J Dent Res. 1994; 73:1397–1406.
Article19. Marhuenda E, Alarcón de la Lastra C, Martín MJ. Antisecretory and gastroprotective effects of aescine in rats. Gen Pharmacol. 1994; 25:1213–1219.
Article20. Martuscelli G, Fiorellini JP, Crohin CC, Howell TH. The effect of interleukin-11 on the progression of ligature-induced periodontal disease in the beagle dog. J Periodontol. 2000; 71:573–578.
Article21. Page RC. Gingivitis. J Clin Periodontol. 1986; 13:345–359.
Article22. Plumb DC. Plumb's Veterinary Drug Handbook. 5th ed. Ames: Blackwell;2005. p. 411–415.23. Salvi GE, Lang NP. Host response modulation in the management of periodontal diseases. J Clin Periodontol. 2005; 32:Suppl 6. 108–129.
Article24. Schroeder HE, Lindhe J. Conversion of stable established gingivitis in the dog into destructive periodontitis. Arch Oral Biol. 1975; 20:775–782.
Article25. Schwarz F, Jepsen S, Herten M, Aoki A, Sculean A, Becker J. Immunohistochemical characterization of periodontal wound healing following nonsurgical treatment with fluorescence controlled Er:YAG laser radiation in dogs. Lasers Surg Med. 2007; 39:428–440.
Article26. Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964; 22:121–135.
Article27. Técucianu JF. [Double-blind clinical study of a titrated extract of an unsaponifiable fraction of Zea mays L. on gingival inflammation]. Inf Dent. 1975; 57:21–32.28. Tervahartiala T, Pirilä E, Ceponis A, Maisi P, Salo T, Tuter G, Kallio P, Törnwall J, Srinivas R, Konttinen YT, Sorsa T. The in vivo expression of the collagenolytic matrix metalloproteinases (MMP-2, -8, -13, and -14) and matrilysin (MMP-7) in adult and localized juvenile periodontitis. J Dent Res. 2000; 79:1969–1977.
Article29. Walker JM. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol Biol. 1994; 32:5–8.
Article30. Wattel A, Kamel S, Prouillet C, Petit JP, Lorget F, Offord E, Brazier M. Flavonoid quercetin decreases osteoclastic differentiation induced by RANKL via a mechanism involving NFκB and AP-1. J Cell Biochem. 2004; 92:285–295.
Article31. Wennström JL, Newman HN, MacNeill SR, Killoy WJ, Griffiths GS, Gilliam DG, Krok L, Needleman IG, Weiss G, Garrett S. Utilisation of locally delivered doxycycline in non-surgical treatment of chronic periodontitis. A comparative multi-centre trial of 2 treatment approaches. J Clin Periodontol. 2001; 28:753–761.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of periodontal flap surgery on Matrix metalloproteinases (MMPs) and Tissue inhibitors of matrix metalloproteinase-1 (TIMP-1) levels in gingival crevicular fluids of periodontitis patients
- Expression of Matrix metalloproteinase-1 between Simple Chronic Periodontitis and Type 2 Diabetes associated Chronic Periodontitis on Protein level
- The expressions of inflammatory factors and tissue inhibitor of matrix metalloproteinase-2 in human chronic periodontitis with type 2 diabetes mellitus
- Effect of Garcinia mangostana L. and propolis extracts on the inhibition of inflammation and alveolar bone loss in ligature-induced periodontitis in rats
- Mongolian Gerbil as a Novel Animal Model for Ligature-induced Periodontitis