Nutr Res Pract.
2017 Apr;11(2):97-104. 10.4162/nrp.2017.11.2.97.
Protective effects of an ethanol extract of Angelica keiskei against acetaminophen-induced hepatotoxicity in HepG2 and HepaRG cells
- Affiliations
-
- 1Department of Food Science and Nutrition, Hallym University, 1 Hallymdaehak-gil, Chuncheon, Gangwon 24252, Korea. ijkang@hallym.ac.kr
- 2Department of Food Science and Nutrition, Dongseo University, Busan 47011, Korea.
- 3Center for Efficacy Assessment and Development of Functional Food and Drugs, Hallym University, 1 Hallymdaehak-gil, Chuncheon, Gangwon 24252, Korea. myej4@hallym.ac.kr
- KMID: 2412021
- DOI: http://doi.org/10.4162/nrp.2017.11.2.97
Abstract
- BACKGROUND
/OBJECTIVE: Although Angelica keiskei (AK) has widely been utilized for the purpose of general health improvement among Asian, its functionality and mechanism of action. The aim of this study was to determine the protective effect of ethanol extract of AK (AK-Ex) on acute hepatotoxicity induced by acetaminophen (AAP) in HepG2 human hepatocellular liver carcinoma cells and HepaRG human hepatic progenitor cells.
MATERIALS/METHODS
AK-Ex was prepared HepG2 and HepaRG cells were cultured with various concentrations and 30 mM AAP. The protective effects of AK-Ex against AAP-induced hepatotoxicity in HepG2 and HepaRG cells were evaluated using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide, lactate dehydrogenase (LDH) assay, flow cytometry, and Western blotting.
RESULTS
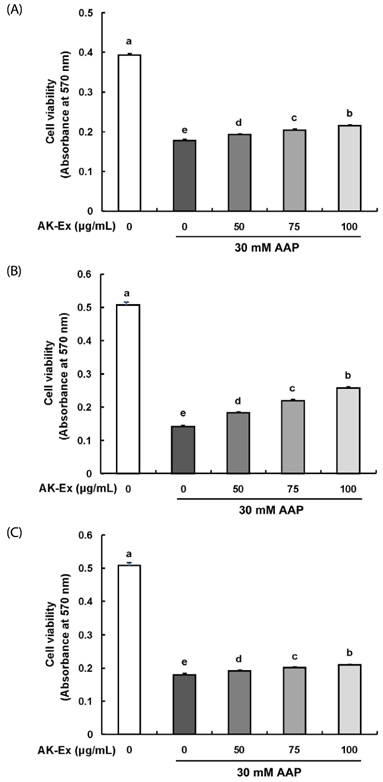
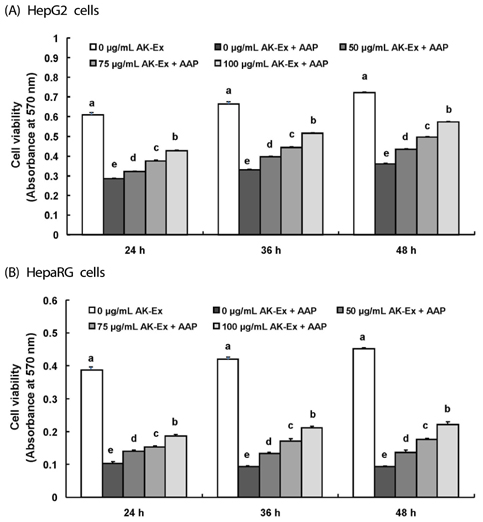
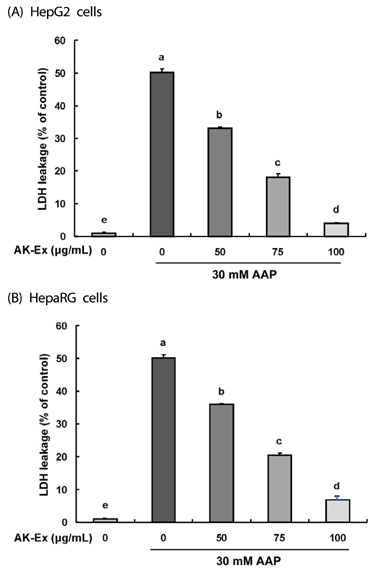
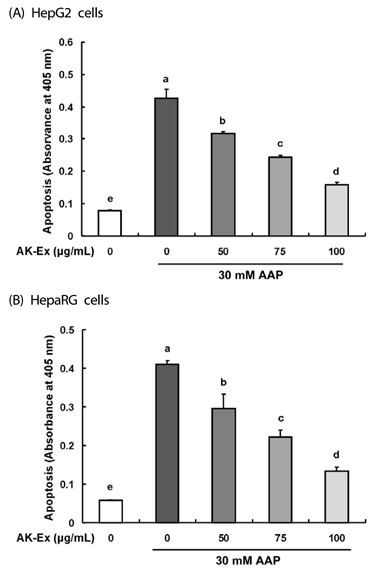
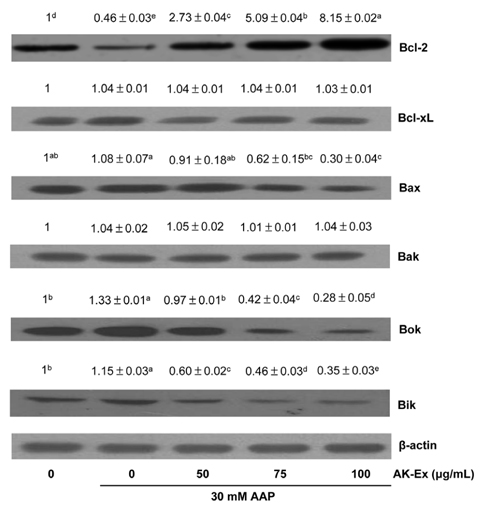
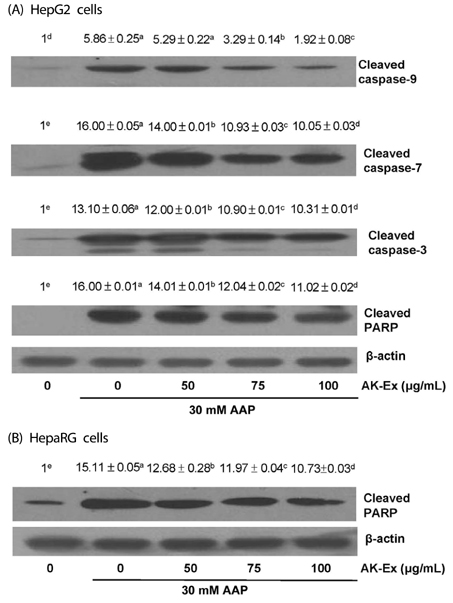
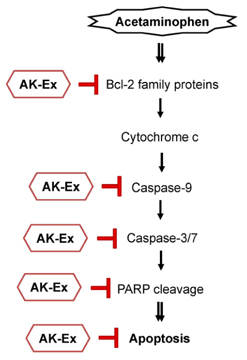
AK-Ex, when administered prior to AAP, increased cell growth and decreased leakage of LDH in a dose-dependent manner in HepG2 and HepaRG cells against AAP-induced hepatotoxicity. AK-Ex increased the level of Bcl-2 and decreased the levels of Bax, Bok and Bik decreased the permeability of the mitochondrial membrane in HepG2 cells intoxicated with AAP. AK-Ex decreased the cleavage of poly (ADP-ribose) polymerase (PARP) and the activation of caspase-9, -7, and -3.
CONCLUSIONS
These results demonstrate that AK-Ex downregulates apoptosis via intrinsic and extrinsic pathways against AAP-induced hepatotoxicity. We suggest that AK could be a useful preventive agent against AAP-induced apoptosis in hepatocytes.
Keyword
MeSH Terms
Figure
Reference
-
1. Maronpot RR. Toxicological assessment of Ashitaba Chalcone. Food Chem Toxicol. 2015; 77:111–119.
Article2. Chang HR, Lee HJ, Ryu JH. Chalcones from Angelica keiskei attenuate the inflammatory responses by suppressing nuclear translocation of NF-kappaB. J Med Food. 2014; 17:1306–1313.
Article3. Yasuda M, Kawabata K, Miyashita M, Okumura M, Yamamoto N, Takahashi M, Ashida H, Ohigashi H. Inhibitory effects of 4-hydroxyderricin and xanthoangelol on lipopolysaccharide-induced inflammatory responses in RAW264 macrophages. J Agric Food Chem. 2014; 62:462–467.
Article4. Zhang T, Yamashita Y, Yasuda M, Yamamoto N, Ashida H. Ashitaba (Angelica keiskei) extract prevents adiposity in high-fat diet-fed C57BL/6 mice. Food Funct. 2015; 6:135–145.
Article5. Ogawa H, Okada Y, Kamisako T, Baba K. Beneficial effect of xanthoangelol, a chalcone compound from Angelica keiskei, on lipid metabolism in stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2007; 34:238–243.
Article6. Kim E, Choi J, Yeo I. The effects of Angelica keiskei Koidz on the expression of antioxidant enzymes related to lipid profiles in rats fed a high fat diet. Nutr Res Pract. 2012; 6:9–15.
Article7. Akihisa T, Kikuchi T, Nagai H, Ishii K, Tabata K, Suzuki T. 4-Hydroxyderricin from Angelica keiskei roots induces caspasedependent apoptotic cell death in HL60 human leukemia cells. J Oleo Sci. 2011; 60:71–77.
Article8. Noh HM, Ahn EM, Yun JM, Cho BL, Paek YJ. Angelica keiskei Koidzumi extracts improve some markers of liver function in habitual alcohol drinkers: a randomized double-blind clinical trial. J Med Food. 2015; 18:166–172.
Article9. Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012; 55:222–232.
Article10. Labbe G, Pessayre D, Fromenty B. Drug-induced liver injury through mitochondrial dysfunction: mechanisms and detection during preclinical safety studies. Fundam Clin Pharmacol. 2008; 22:335–353.
Article11. Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, Lee WM. Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005; 42:1364–1372.
Article12. Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990; 255:935–941.13. Holownia A, Braszko JJ. Acetaminophen alters microsomal ryanodine Ca2+ channel in HepG2 cells overexpressing CYP2E1. Biochem Pharmacol. 2004; 68:513–521.
Article14. Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004; 40:1170–1179.
Article15. Kim EJ, Holthuizen PE, Park HS, Ha YL, Jung KC, Park JH. Trans-10, cis-12-conjugated linoleic acid inhibits Caco-2 colon cancer cell growth. Am J Physiol Gastrointest Liver Physiol. 2002; 283:G357–G367.16. Thomas JP, Geiger PG, Girotti AW. Lethal damage to endothelial cells by oxidized low density lipoprotein: role of selenoperoxidases in cytoprotection against lipid hydroperoxide- and iron-mediated reactions. J Lipid Res. 1993; 34:479–490.
Article17. Jung JI, Lim SS, Choi HJ, Cho HJ, Shin HK, Kim EJ, Chung WY, Park KK, Park JH. Isoliquiritigenin induces apoptosis by depolarizing mitochondrial membranes in prostate cancer cells. J Nutr Biochem. 2006; 17:689–696.
Article18. Cho HJ, Kim WK, Kim EJ, Jung KC, Park S, Lee HS, Tyner AL, Park JH. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am J Physiol Gastrointest Liver Physiol. 2003; 284:G996–1005.19. Jetten MJ, Kleinjans JC, Claessen SM, Chesne C, van Delft JH. Baseline and genotoxic compound induced gene expression profiles in HepG2 and HepaRG compared to primary human hepatocytes. Toxicol In Vitro. 2013; 27:2031–2040.
Article20. Jennen DG, Magkoufopoulou C, Ketelslegers HB, van Herwijnen MH, Kleinjans JC, van Delft JH. Comparison of HepG2 and HepaRG by whole-genome gene expression analysis for the purpose of chemical hazard identification. Toxicol Sci. 2010; 115:66–79.
Article21. Liguori MJ, Blomme EA, Waring JF. Trovafloxacin-induced gene expression changes in liver-derived in vitro systems: comparison of primary human hepatocytes to HepG2 cells. Drug Metab Dispos. 2008; 36:223–233.
Article22. Guo L, Dial S, Shi L, Branham W, Liu J, Fang JL, Green B, Deng H, Kaput J, Ning B. Similarities and differences in the expression of drug-metabolizing enzymes between human hepatic cell lines and primary human hepatocytes. Drug Metab Dispos. 2011; 39:528–538.
Article23. Aninat C, Piton A, Glaise D, Le Charpentier T, Langouët S, Morel F, Guguen-Guillouzo C, Guillouzo A. Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab Dispos. 2006; 34:75–83.
Article24. Andersson TB. The application of HepRG cells in evaluation of cytochrome P450 induction properties of drug compounds. Methods Mol Biol. 2010; 640:375–387.
Article25. Ferreira A, Rodrigues M, Silvestre S, Falcão A, Alves G. HepaRG cell line as an in vitro model for screening drug-drug interactions mediated by metabolic induction: amiodarone used as a model substance. Toxicol In Vitro. 2014; 28:1531–1535.
Article26. Danpure CJ. Lactate dehydrogenase and cell injury. Cell Biochem Funct. 1984; 2:144–148.
Article27. Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003; 22:8590–8607.
Article28. Ohkura N, Nakakuki Y, Taniguchi M, Kanai S, Nakayama A, Ohnishi K, Sakata T, Nohira T, Matsuda J, Baba K, Atsumi G. Xanthoangelols isolated from Angelica keiskei inhibit inflammatory-induced plasminogen activator inhibitor 1 (PAI-1) production. Biofactors. 2011; 37:455–461.
Article29. Nagata J, Morino T, Saito M. Effects of dietary Angelica keiskei on serum and liver lipid profiles, and body fat accumulations in rats. J Nutr Sci Vitaminol (Tokyo). 2007; 53:133–137.
Article30. Aoki N, Muko M, Ohta E, Ohta S. C-geranylated chalcones from the stems of Angelica keiskei with superoxide-scavenging activity. J Nat Prod. 2008; 71:1308–1310.
Article31. Ohnogi H, Hayami S, Kudo Y, Deguchi S, Mizutani S, Enoki T, Tanimura Y, Aoi W, Naito Y, Kato I, Yoshikawa T. Angelica keiskei extract improves insulin resistance and hypertriglyceridemia in rats fed a high-fructose drink. Biosci Biotechnol Biochem. 2012; 76:928–932.
Article32. Kawabata K, Sawada K, Ikeda K, Fukuda I, Kawasaki K, Yamamoto N, Ashida H. Prenylated chalcones 4-hydroxyderricin and xanthoangelol stimulate glucose uptake in skeletal muscle cells by inducing GLUT4 translocation. Mol Nutr Food Res. 2011; 55:467–475.
Article33. Oh SR, Kim SJ, Kim DH, Ryu JH, Ahn EM, Jung JW. Angelica keiskei ameliorates scopolamine-induced memory impairments in mice. Biol Pharm Bull. 2013; 36:82–88.
Article34. Choi SH, Park KH. Protective effects of Angelica keiskei extracts against D-galactosamine(GalN)-induced hepatotoxicity in rats. J Food Hyg Saf. 2011; 26:235–241.35. Huerta S, Goulet EJ, Huerta-Yepez S, Livingston EH. Screening and detection of apoptosis. J Surg Res. 2007; 139:143–156.
Article36. Thomadaki H, Scorilas A. BCL2 family of apoptosis-related genes: functions and clinical implications in cancer. Crit Rev Clin Lab Sci. 2006; 43:1–67.
Article37. Boise LH, González-García M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nuñez G, Thompson CB. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993; 74:597–608.
Article38. Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999; 6:1028–1042.
Article39. Bürkle A, Brabeck C, Diefenbach J, Beneke S. The emerging role of poly(ADP-ribose) polymerase-1 in longevity. Int J Biochem Cell Biol. 2005; 37:1043–1053.
Article40. Andrabi SA, Kim NS, Yu SW, Wang H, Koh DW, Sasaki M, Klaus JA, Otsuka T, Zhang Z, Koehler RC, Hurn PD, Poirier GG, Dawson VL, Dawson TM. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci U S A. 2006; 103:18308–18313.
Article41. Isabelle M, Moreel X, Gagné JP, Rouleau M, Ethier C, Gagné P, Hendzel MJ, Poirier GG. Investigation of PARP-1, PARP-2, and PARG interactomes by affinity-purification mass spectrometry. Proteome Sci. 2010; 8:22.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Evaluation of Metabolizable Energy of Angelica Keiskei (Angelica utilis Makino) Products
- Comparison of in vitro models for drug-induced liver injury assessment
- Two Faces of "Green Juice"
- Protective effect of chlorophyllremoved ethanol extract of Lycium barbarum leaves against nonalcoholic fatty liver disease
- Ibuprofen Increases the Hepatotoxicity of Ethanol through Potentiating Oxidative Stress