Korean J Radiol.
2018 Jun;19(3):452-462. 10.3348/kjr.2018.19.3.452.
Brain Regional Homogeneity Changes in Cirrhotic Patients with or without Hepatic Encephalopathy Revealed by Multi-Frequency Bands Analysis Based on Resting-State Functional MRI
- Affiliations
-
- 1Tianjin Key Laboratory of Cognitive Computing and Application, School of Computer Science and Technology, Tianjin University, Tianjin 300350, China.
- 2Department of Radiology, Tianjin First Central Hospital, Tianjin 300192, China. chengyue200017076@163.com
- 3State Key Laboratory of Intelligent Technology and Systems, National Laboratory for Information Science and Technology, Tsinghua University, Beijing 100084, China.
- KMID: 2410816
- DOI: http://doi.org/10.3348/kjr.2018.19.3.452
Abstract
OBJECTIVE
To investigate brain regional homogeneity (ReHo) changes of multiple sub-frequency bands in cirrhotic patients with or without hepatic encephalopathy using resting-state functional MRI.
MATERIALS AND METHODS
This study recruited 46 cirrhotic patients without clinical hepatic encephalopathy (noHE), 38 cirrhotic patients with clinical hepatic encephalopathy (HE), and 37 healthy volunteers. ReHo differences were analyzed in slow-5 (0.010−0.027 Hz), slow-4 (0.027−0.073 Hz), and slow-3 (0.073−0.198 Hz) bands. Routine analysis of (0.010−0.080 Hz) band was used as a benchmark. Associations of abnormal ReHo values in each frequency band with neuropsychological scores and blood ammonia level were analyzed. Pattern classification analyses were conducted to determine whether ReHo differences in each band could differentiate the three groups of subjects (patients with or without hepatic encephalopathy and healthy controls).
RESULTS
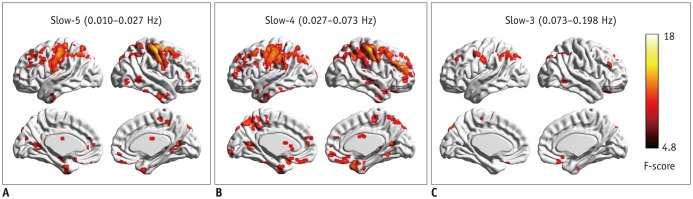
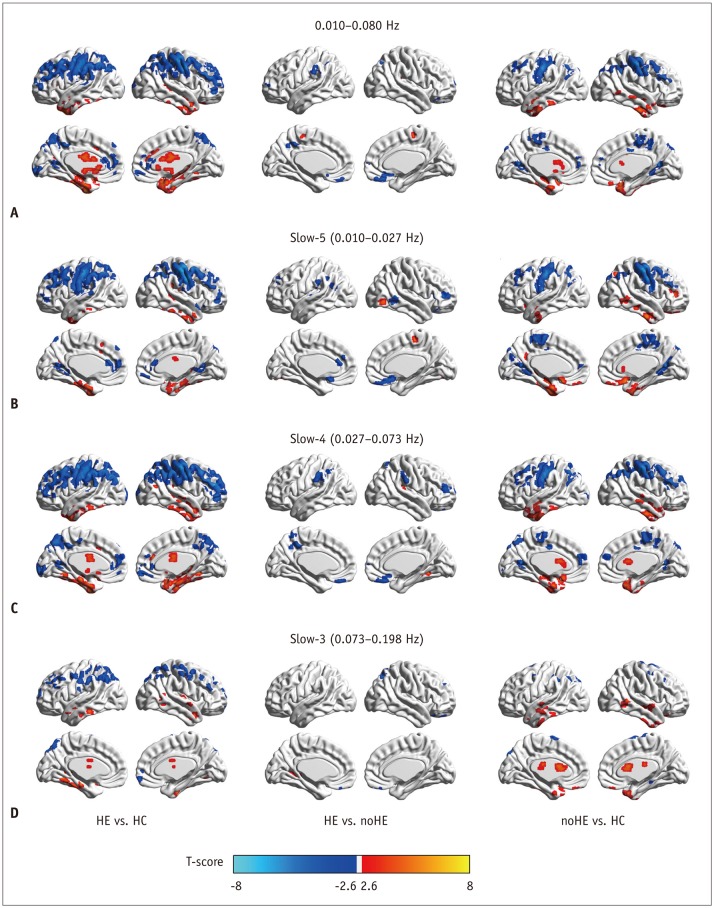
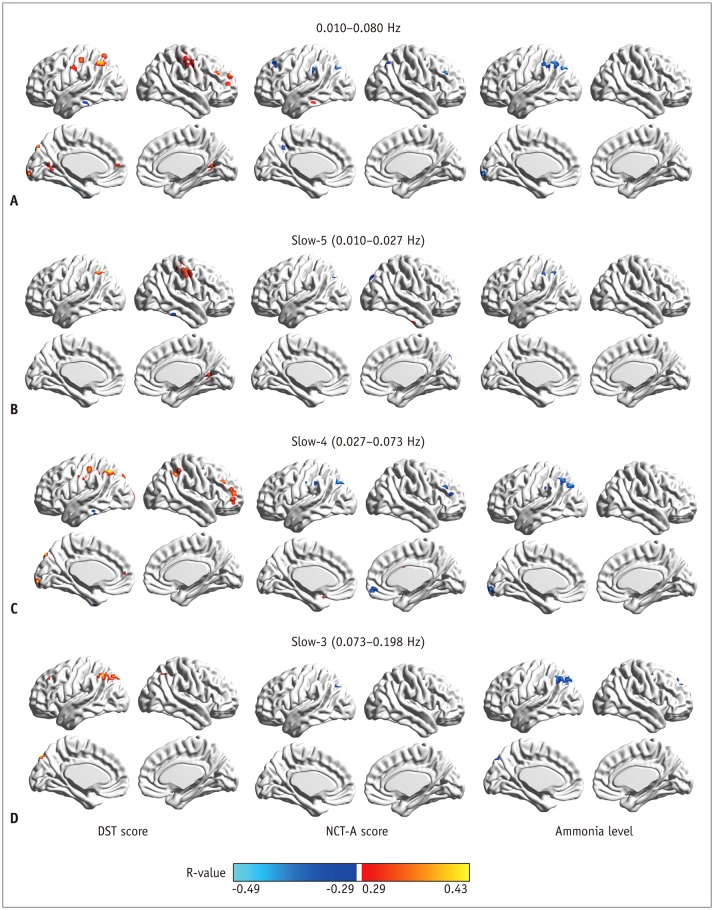
Compared to routine analysis, more differences between HE and noHE were observed in slow-5 and slow-4 bands (p < 0.005, cluster > 12, overall corrected p < 0.05). Sub-frequency band analysis also showed that ReHo abnormalities were frequency-dependent (overall corrected p < 0.05). In addition, ReHo abnormalities in each sub-band were correlated with blood ammonia level and neuropsychological scores, especially in the left inferior parietal lobe (overall corrected p < 0.05 for all frequency bands). Pattern classification analysis demonstrated that ReHo differences in lower slow-5 and slow-4 bands (both p < 0.05) and higher slow-3 band could differentiate the three groups (p < 0.05). Compared to routine analysis, ReHo features in slow-4 band obtained better classification accuracy (89%).
CONCLUSION
Cirrhotic patients showed frequency-dependent changes in ReHo. Sub-frequency band analysis is important for understanding HE and clinical monitoring.
Keyword
MeSH Terms
Figure
Reference
-
1. Hsu TW, Wu CW, Cheng YF, Chen HL, Lu CH, Cho KH, et al. Impaired small-world network efficiency and dynamic functional distribution in patients with cirrhosis. PLoS One. 2012; 7:e35266. PMID: 22563460.
Article2. Alonso J, Córdoba J, Rovira A. Brain magnetic resonance in hepatic encephalopathy. Semin Ultrasound CT MR. 2014; 35:136–152. PMID: 24745889.
Article3. Cheng Y, Zhang G, Shen W, Huang LX, Zhang L, Xie SS, et al. Impact of previous episodes of hepatic encephalopathy on short-term brain function recovery after liver transplantation: a functional connectivity strength study. Metab Brain Dis. 2018; 33:237–249. PMID: 29170933.
Article4. McPhail MJ, Patel NR, Taylor-Robinson SD. Brain imaging and hepatic encephalopathy. Clin Liver Dis. 2012; 16:57–72. PMID: 22321465.
Article5. Park SH, Han PK, Choi SH. Physiological and functional magnetic resonance imaging using balanced steady-state free precession. Korean J Radiol. 2015; 16:550–559. PMID: 25995684.
Article6. Lv XF, Ye M, Han LJ, Zhang XL, Cai PQ, Jiang GH, et al. Abnormal baseline brain activity in patients with HBV-related cirrhosis without overt hepatic encephalopathy revealed by resting-state functional MRI. Metab Brain Dis. 2013; 28:485–492. PMID: 23836055.
Article7. Chen HJ, Zhu XQ, Jiao Y, Li PC, Wang Y, Teng GJ. Abnormal baseline brain activity in low-grade hepatic encephalopathy: a resting-state fMRI study. J Neurol Sci. 2012; 318:140–145. PMID: 22541365.
Article8. Zhang G, Cheng Y, Shen W, Liu B, Huang L, Xie S. The shortterm effect of liver transplantation on the low-frequency fluctuation of brain activity in cirrhotic patients with and without overt hepatic encephalopathy. Brain Imaging Behav. 2017; 11:1849–1861. PMID: 27917450.
Article9. Zhang G, Cheng Y, Liu B. Abnormalities of voxel-based wholebrain functional connectivity patterns predict the progression of hepatic encephalopathy. Brain Imaging Behav. 2017; 11:784–796. PMID: 27138528.
Article10. Chen HJ, Zhu XQ, Shu H, Yang M, Zhang Y, Ding J, et al. Structural and functional cerebral impairments in cirrhotic patients with a history of overt hepatic encephalopathy. Eur J Radiol. 2012; 81:2463–2469. PMID: 22056487.
Article11. Lv XF, Qiu YW, Tian JZ, Xie CM, Han LJ, Su HH, et al. Abnormal regional homogeneity of resting-state brain activity in patients with HBV-related cirrhosis without overt hepatic encephalopathy. Liver Int. 2013; 33:375–383. PMID: 23402608.
Article12. Lin WC, Hsu TW, Chen CL, Lu CH, Chen HL, Cheng YF. Resting state-fMRI with ReHo analysis as a non-invasive modality for the prognosis of cirrhotic patients with overt hepatic encephalopathy. PLoS One. 2015; 10:e0126834. PMID: 25973853.
Article13. Song X, Zhou S, Zhang Y, Liu Y, Zhu H, Gao JH. Frequencydependent modulation of regional synchrony in the human brain by eyes open and eyes closed resting-states. PLoS One. 2015; 10:e0141507. PMID: 26545233.
Article14. Qian L, Zhang Y, Zheng L, Shang Y, Gao JH, Liu Y. Frequency dependent topological patterns of resting-state brain networks. PLoS One. 2015; 10:e0124681. PMID: 25927525.
Article15. Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, et al. The oscillating brain: complex and reliable. Neuroimage. 2010; 49:1432–1445. PMID: 19782143.
Article16. He BJ, Zempel JM, Snyder AZ, Raichle ME. The temporal structures and functional significance of scale-free brain activity. Neuron. 2010; 66:353–369. PMID: 20471349.
Article17. Baria AT, Baliki MN, Parrish T, Apkarian AV. Anatomical and functional assemblies of brain BOLD oscillations. J Neurosci. 2011; 31:7910–7919. PMID: 21613505.
Article18. Wang P, Li R, Yu J, Huang Z, Li J. Frequency-dependent brain regional homogeneity alterations in patients with mild cognitive impairment during working memory state relative to resting state. Front Aging Neurosci. 2016; 8:60. PMID: 27047375.
Article19. Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004; 304:1926–1929. PMID: 15218136.
Article20. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973; 60:646–649. PMID: 4541913.
Article21. Atterbury CE, Maddrey WC, Conn HO. Neomycin-sorbitol and lactulose in the treatment of acute portal-systemic encephalopathy. A controlled, double-blind clinical trial. Am J Dig Dis. 1978; 23:398–406. PMID: 354373.22. Cheng Y, Huang L, Zhang X, Zhong J, Ji Q, Xie S, et al. Liver transplantation nearly normalizes brain spontaneous activity and cognitive function at 1 month: a resting-state functional MRI study. Metab Brain Dis. 2015; 30:979–988. PMID: 25703240.23. Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 2016; 14:339–351. PMID: 27075850.24. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002; 17:825–841. PMID: 12377157.
Article25. Song X, Zhang Y, Liu Y. Frequency specificity of regional homogeneity in the resting-state human brain. PLoS One. 2014; 9:e86818. PMID: 24466256.
Article26. Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004; 22:394–400. PMID: 15110032.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Renormalization of Thalamic Sub-Regional Functional Connectivity Contributes to Improvement of Cognitive Function after Liver Transplantation in Cirrhotic Patients with Overt Hepatic Encephalopathy
- Alterations in Spontaneous Brain Activity in Drug-Naïve First-Episode Schizophrenia: An Anatomical/Activation Likelihood Estimation Meta-Analysis
- Resting-State Electroencephalography (EEG) Functional Connectivity Analysis
- Regional Homogeneity Brain Alterations in Schizophrenia: An Activation Likelihood Estimation Meta-Analysis
- Proton Magnetic Resonance Spectroscopic Findings in Cirrhotic Patients with Hepatic Encephalopathy : The Significance of Decreased Myoinositol / Creatine Ratio