Korean J Physiol Pharmacol.
2018 May;22(3):321-329. 10.4196/kjpp.2018.22.3.321.
Physiologically-based pharmacokinetic predictions of intestinal BCRP-mediated drug interactions of rosuvastatin in Koreans
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul St. Mary's Hospital, Seoul 06591, Korea. yimds@catholic.ac.kr
- 2PIPET (Pharmacometrics Institute for Practical Education and Training), College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- 3Department of Clinical Pharmacology, Severance Hospital, Yonsei University College of Medicine, Seoul 03722, Korea.
- 4Q-fitter Inc., Seoul 06199, Korea.
- KMID: 2410096
- DOI: http://doi.org/10.4196/kjpp.2018.22.3.321
Abstract
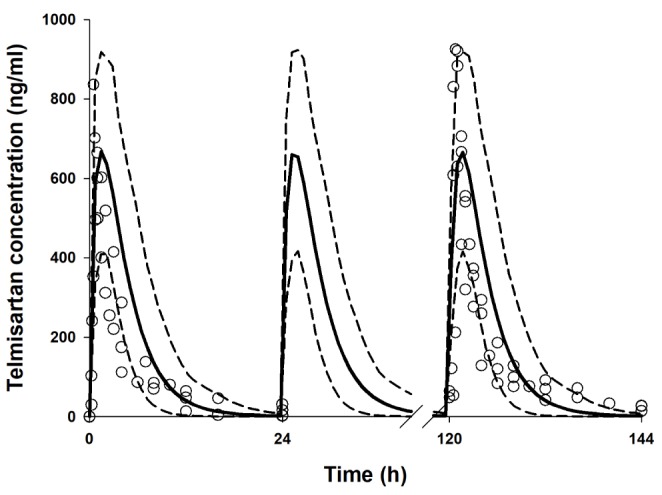
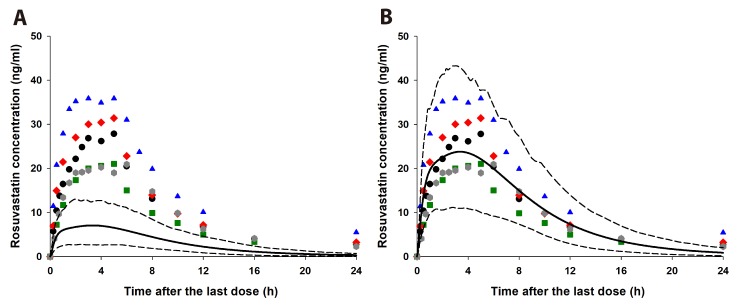
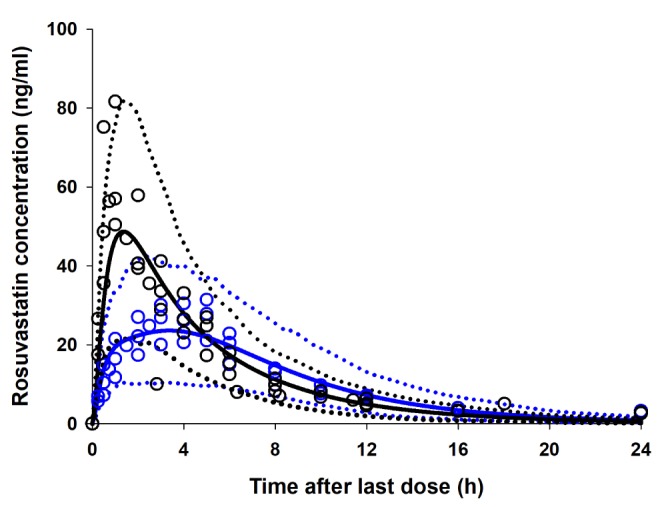
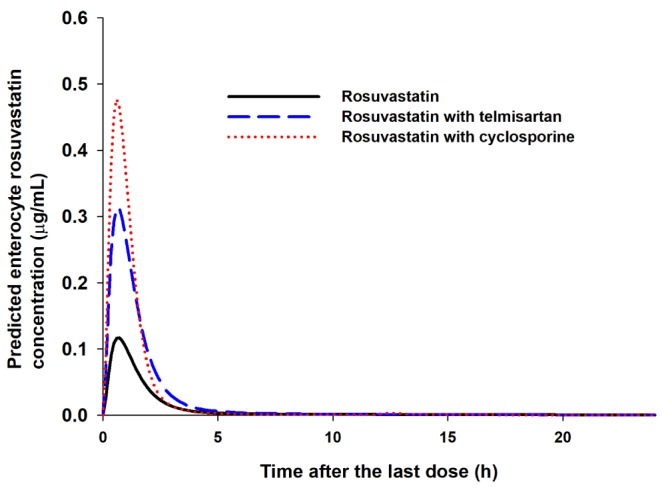
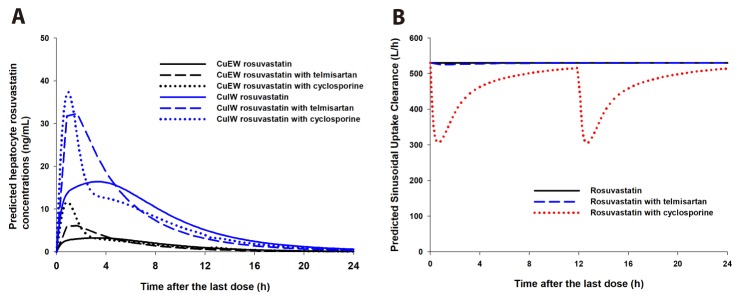
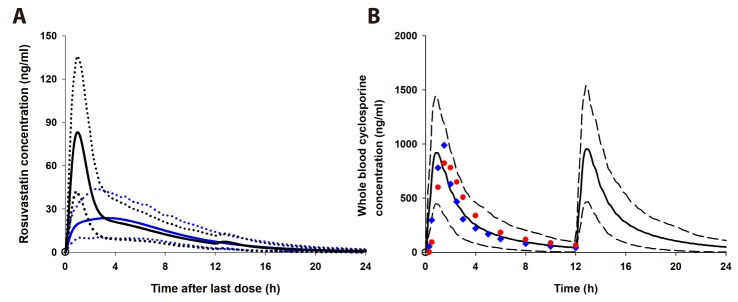
- It was recently reported that the C(max) and AUC of rosuvastatin increases when it is coadministered with telmisartan and cyclosporine. Rosuvastatin is known to be a substrate of OATP1B1, OATP1B3, NTCP, and BCRP transporters. The aim of this study was to explore the mechanism of the interactions between rosuvastatin and two perpetrators, telmisartan and cyclosporine. Published (cyclosporine) or newly developed (telmisartan) PBPK models were used to this end. The rosuvastatin model in Simcyp (version 15)'s drug library was modified to reflect racial differences in rosuvastatin exposure. In the telmisartan-rosuvastatin case, simulated rosuvastatin C(maxI)/C(max) and AUC(I)/AUC (with/without telmisartan) ratios were 1.92 and 1.14, respectively, and the T(max) changed from 3.35 h to 1.40 h with coadministration of telmisartan, which were consistent with the aforementioned report (C(maxI)/C(max): 2.01, AUCI/AUC:1.18, T(max): 5 h → 0.75 h). In the next case of cyclosporine-rosuvastatin, the simulated rosuvastatin C(maxI)/C(max) and AUC(I)/AUC (with/without cyclosporine) ratios were 3.29 and 1.30, respectively. The decrease in the CL(int,BCRP,intestine) of rosuvastatin by telmisartan and cyclosporine in the PBPK model was pivotal to reproducing this finding in Simcyp. Our PBPK model demonstrated that the major causes of increase in rosuvastatin exposure are mediated by intestinal BCRP (rosuvastatin-telmisartan interaction) or by both of BCRP and OATP1B1/3 (rosuvastatin-cyclosporine interaction).
Keyword
MeSH Terms
Figure
Reference
-
1. Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008; 117:e25–e146. PMID: 18086926.2. Johnson ML, Pietz K, Battleman DS, Beyth RJ. Prevalence of comorbid hypertension and dyslipidemia and associated cardiovascular disease. Am J Manag Care. 2004; 10:926–932. PMID: 15617368.3. Son M, Kim Y, Lee D, Roh H, Son H, Guk J, Jang SB, Nam SY, Park K. Pharmacokinetic interaction between rosuvastatin and telmisartan in healthy Korean male volunteers: a randomized, open-label, two-period, crossover, multiple-dose study. Clin Ther. 2014; 36:1147–1158. PMID: 24998012.
Article4. Jamei M, Bajot F, Neuhoff S, Barter Z, Yang J, Rostami-Hodjegan A, Rowland-Yeo K. A mechanistic framework for in vitro-in vivo extrapolation of liver membrane transporters: prediction of drug-drug interaction between rosuvastatin and cyclosporine. Clin Pharmacokinet. 2014; 53:73–87. PMID: 23881596.
Article5. Simonson SG, Raza A, Martin PD, Mitchell PD, Jarcho JA, Brown CD, Windass AS, Schneck DW. Rosuvastatin pharmacokinetics in heart transplant recipients administered an antirejection regimen including cyclosporine. Clin Pharmacol Ther. 2004; 76:167–177. PMID: 15289793.
Article6. Oliverio MI, Coffman TM. Angiotensin-II-receptors: new targets for antihypertensive therapy. Clin Cardiol. 1997; 20:3–6. PMID: 8994730.7. Ishiguro N, Maeda K, Kishimoto W, Saito A, Harada A, Ebner T, Roth W, Igarashi T, Sugiyama Y. Predominant contribution of OATP1B3 to the hepatic uptake of telmisartan, an angiotensin II receptor antagonist, in humans. Drug Metab Dispos. 2006; 34:1109–1115. PMID: 16611857.
Article8. Ieiri I, Nishimura C, Maeda K, Sasaki T, Kimura M, Chiyoda T, Hirota T, Irie S, Shimizu H, Noguchi T, Yoshida K, Sugiyama Y. Pharmacokinetic and pharmacogenomic profiles of telmisartan after the oral microdose and therapeutic dose. Pharmacogenet Genomics. 2011; 21:495–505. PMID: 21691256.
Article9. Stangier J, Schmid J, Türck D, Switek H, Verhagen A, Peeters PA, van Marle SP, Tamminga WJ, Sollie FA, Jonkman JH. Absorption, metabolism, and excretion of intravenously and orally administered [14C]telmisartan in healthy volunteers. J Clin Pharmacol. 2000; 40:1312–1322. PMID: 11185629.10. Hirano M, Maeda K, Shitara Y, Sugiyama Y. Drug-drug interaction between pitavastatin and various drugs via OATP1B1. Drug Metab Dispos. 2006; 34:1229–1236. PMID: 16595711.
Article11. Zhang A, Wang C, Liu Q, Meng Q, Peng J, Sun H, Ma X, Huo X, Liu K. Involvement of organic anion-transporting polypeptides in the hepatic uptake of dioscin in rats and humans. Drug Metab Dispos. 2013; 41:994–1003. PMID: 23396419.
Article12. Deppe S, Ripperger A, Weiss J, Ergün S, Benndorf RA. Impact of genetic variability in the ABCG2 gene on ABCG2 expression, function, and interaction with AT1 receptor antagonist telmisartan. Biochem Biophys Res Commun. 2014; 443:1211–1217. PMID: 24388985.
Article13. Weiss J, Sauer A, Divac N, Herzog M, Schwedhelm E, Böger RH, Haefeli WE, Benndorf RA. Interaction of angiotensin receptor type 1 blockers with ATP-binding cassette transporters. Biopharm Drug Dispos. 2010; 31:150–161. PMID: 20222053.
Article14. Rubba P, Marotta G, Gentile M. Efficacy and safety of rosuvastatin in the management of dyslipidemia. Vasc Health Risk Manag. 2009; 5:343–352. PMID: 19436657.
Article15. Martin PD, Mitchell PD, Schneck DW. Pharmacodynamic effects and pharmacokinetics of a new HMG-CoA reductase inhibitor, rosuvastatin, after morning or evening administration in healthy volunteers. Br J Clin Pharmacol. 2002; 54:472–477. PMID: 12445025.
Article16. Martin PD, Warwick MJ, Dane AL, Brindley C, Short T. Absolute oral bioavailability of rosuvastatin in healthy white adult male volunteers. Clin Ther. 2003; 25:2553–2563. PMID: 14667956.
Article17. White CM. A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol. 2002; 42:963–970. PMID: 12211221.
Article18. Bergman E, Forsell P, Tevell A, Persson EM, Hedeland M, Bondesson U, Knutson L, Lennernäs H. Biliary secretion of rosuvastatin and bile acids in humans during the absorption phase. Eur J Pharm Sci. 2006; 29:205–214. PMID: 16806856.
Article19. Kitamura S, Maeda K, Wang Y, Sugiyama Y. Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab Dispos. 2008; 36:2014–2023. PMID: 18617601.
Article20. Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, Wang Y, Kim RB. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006; 130:1793–1806. PMID: 16697742.
Article21. Lee E, Ryan S, Birmingham B, Zalikowski J, March R, Ambrose H, Moore R, Lee C, Chen Y, Schneck D. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther. 2005; 78:330–341. PMID: 16198652.
Article22. Hu M, To KK, Mak VW, Tomlinson B. The ABCG2 transporter and its relations with the pharmacokinetics, drug interaction and lipid-lowering effects of statins. Expert Opin Drug Metab Toxicol. 2011; 7:49–62. PMID: 21091277.
Article23. Ieiri I, Higuchi S, Sugiyama Y. Genetic polymorphisms of uptake (OATP1B1, 1B3) and efflux (MRP2, BCRP) transporters: implications for inter-individual differences in the pharmacokinetics and pharmacodynamics of statins and other clinically relevant drugs. Expert Opin Drug Metab Toxicol. 2009; 5:703–729. PMID: 19442037.
Article24. Elsby R, Martin P, Surry D, Sharma P, Fenner K. Solitary inhibition of the breast cancer resistance protein efflux transporter results in a clinically significant drug-drug interaction with rosuvastatin by causing up to a 2-fold increase in statin exposure. Drug Metab Dispos. 2016; 44:398–408. PMID: 26700956.
Article25. Hu M, Lee HK, To KK, Fok BS, Wo SK, Ho CS, Wong CK, Zuo Z, Chan TY, Chan JC, Tomlinson B. Telmisartan increases systemic exposure to rosuvastatin after single and multiple doses, and in vitro studies show telmisartan inhibits ABCG2-mediated transport of rosuvastatin. Eur J Clin Pharmacol. 2016; 72:1471–1478. PMID: 27651239.
Article26. Kim KT, Birmingham BK, Azumaya CT, Chen Y, Schneck D, Zalikowski J. Increased systemic exposure to rosuvastatin in Asian subjects residing in the United States compared to Caucasian subjects. Clin Pharmacol Ther. 2008; 83:S14.27. US Food and Drug Aministration. Clinical pharmacology and biopharmaceutics review. Rockville, MD: US Food and Drug Aministration;2006. p. 15. Report No.: NDA 21-366 Crestor.28. Li R, Ghosh A, Maurer TS, Kimoto E, Barton HA. Physiologically based pharmacokinetic prediction of telmisartan in human. Drug Metab Dispos. 2014; 42:1646–1655. PMID: 25092714.
Article29. Yang J, Jamei M, Yeo KR, Tucker GT, Rostami-Hodjegan A. Prediction of intestinal first-pass drug metabolism. Curr Drug Metab. 2007; 8:676–684. PMID: 17979655.
Article30. Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006; 95:1238–1257. PMID: 16639716.
Article31. Son H, Roh H, Lee D, Chang H, Kim J, Yun C, Park K. Pharmacokinetics of rosuvastatin/olmesartan fixed-dose combination: a singledose, randomized, open-label, 2-period crossover study in healthy Korean subjects. Clin Ther. 2013; 35:915–922. PMID: 23810276.
Article32. Park WS, Jang D, Han S, Yim DS. Mixed-effects analysis of increased rosuvastatin absorption by coadministered telmisartan. Transl Clin Pharmacol. 2016; 24:55–62.
Article33. Cer RZ, Mudunuri U, Stephens R, Lebeda FJ. IC50-to-Ki: a webbased tool for converting IC50 to Ki values for inhibitors of enzyme activity and ligand binding. Nucleic Acids Res. 2009; 37:W441–W445. PMID: 19395593.
Article34. Lim MS, Seong SJ, Park J, Seo JJ, Lee J, Yu KS, Lee HW, Yoon YR. Assessment of pharmacokinetic proportionality of levofloxacin and cyclosporine over a 100-fold dose range in healthy human volunteers. Expert Opin Drug Metab Toxicol. 2012; 8:399–405. PMID: 22404324.
Article35. Oliveira-Freitas VL, Dalla Costa T, Manfro RC, Cruz LB, Schwartsmann G. Influence of purple grape juice in cyclosporine bioavailability. J Ren Nutr. 2010; 20:309–313. PMID: 20303792.36. Yamada A, Maeda K, Ishiguro N, Tsuda Y, Igarashi T, Ebner T, Roth W, Ikushiro S, Sugiyama Y. The impact of pharmacogenetics of metabolic enzymes and transporters on the pharmacokinetics of telmisartan in healthy volunteers. Pharmacogenet Genomics. 2011; 21:523–530. PMID: 21829131.
Article37. Zhang P, Zhang Y, Chen X, Li R, Yin J, Zhong D. Pharmacokinetics of telmisartan in healthy Chinese subjects after oral administration of two dosage levels. Arzneimittelforschung. 2006; 56:569–573. PMID: 17009837.
Article38. Stangier J, Su CA, Roth W. Pharmacokinetics of orally and intravenously administered telmisartan in healthy young and elderly volunteers and in hypertensive patients. J Int Med Res. 2000; 28:149–167. PMID: 11014323.
Article39. Noh YH, Lim HS, Kim MJ, Kim YH, Choi HY, Sung HR, Jin SJ, Lim J, Bae KS. Pharmacokinetic interaction of telmisartan with samlodipine: an open-label, two-period crossover study in healthy Korean male volunteers. Clin Ther. 2012; 34:1625–1635. PMID: 22721873.
Article40. Birmingham BK, Bujac SR, Elsby R, Azumaya CT, Zalikowski J, Chen Y, Kim K, Ambrose HJ. Rosuvastatin pharmacokinetics and pharmacogenetics in Caucasian and Asian subjects residing in the United States. Eur J Clin Pharmacol. 2015; 71:329–340. PMID: 25630984.
Article41. Harwood MD, Achour B, Neuhoff S, Russell MR, Carlson G, Warhurst G, Rostami-Hodjegan A. In vitro-in vivo extrapolation scaling factors for intestinal P-glycoprotein and breast cancer resistance protein: part II. The impact of cross-laboratory variations of intestinal transporter relative expression factors on predicted drug disposition. Drug Metab Dispos. 2016; 44:476–480. PMID: 26842595.
Article42. Schneck DW, Birmingham BK, Zalikowski JA, Mitchell PD, Wang Y, Martin PD, Lasseter KC, Brown CD, Windass AS, Raza A. The effect of gemfibrozil on the pharmacokinetics of rosuvastatin. Clin Pharmacol Ther. 2004; 75:455–463. PMID: 15116058.
Article43. Pang KS, Rodrigues AD, Peter RM. Enzyme- and transporter-based drugd-drug interactions. New York: Springer;2010. p. 301–307.44. Gill KL, Houston JB, Galetin A. Characterization of in vitro glucuronidation clearance of a range of drugs in human kidney microsomes: comparison with liver and intestinal glucuronidation and impact of albumin. Drug Metab Dispos. 2012; 40:825–835. PMID: 22275465.
Article45. Tomita Y, Maeda K, Sugiyama Y. Ethnic variability in the plasma exposures of OATP1B1 substrates such as HMG-CoA reductase inhibitors: a kinetic consideration of its mechanism. Clin Pharmacol Ther. 2013; 94:37–51. PMID: 23443754.
Article46. Trdan Lušin T, Stieger B, Marc J, Mrhar A, Trontelj J, Zavratnik A, Ostanek B. Organic anion transporting polypeptides OATP1B1 and OATP1B3 and their genetic variants influence the pharmacokinetics and pharmacodynamics of raloxifene. J Transl Med. 2012; 10:76. PMID: 22533838.
Article47. Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011; 63:157–181. PMID: 21245207.
Article48. Li R, Barton HA, Maurer TS. Toward prospective prediction of pharmacokinetics in OATP1B1 genetic variant populations. CPT Pharmacometrics Syst Pharmacol. 2014; 3:e151. PMID: 25494035.
Article49. Elsby R, Hilgendorf C, Fenner K. Understanding the critical disposition pathways of statins to assess drug-drug interaction risk during drug development: it’s not just about OATP1B1. Clin Pharmacol Ther. 2012; 92:584–598. PMID: 23047648.
Article50. Sakiyama M, Matsuo H, Takada Y, Nakamura T, Nakayama A, Takada T, Kitajiri S, Wakai K, Suzuki H, Shinomiya N. Ethnic differences in ATP-binding cassette transporter, sub-family G, member 2 (ABCG2/BCRP): genotype combinations and estimated functions. Drug Metab Pharmacokinet. 2014; 29:490–492. PMID: 24869748.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mixed?effects analysis of increased rosuvastatin absorption by coadministered telmisartan
- Basic Principles of Drug Interaction
- Rosuvastatin-induced Generalized Drug Eruption
- Physiological spaces and multicompartmental pharmacokinetic models
- Prediction of metabolizing enzymemediated clinical drug interactions using in vitro information