Allergy Asthma Immunol Res.
2018 May;10(3):216-224. 10.4168/aair.2018.10.3.216.
TRPV1 Blocking Alleviates Airway Inflammation and Remodeling in a Chronic Asthma Murine Model
- Affiliations
-
- 1Division of Pulmonology, Department of Internal Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea. sooklee@catholic.ac.kr
- 2Division of Pulmonology, Department of Internal Medicine, Uijeongbu St. Mary's Hospital, The Catholic University of Korea College of Medicine, Uijeongbu, Korea.
- KMID: 2409056
- DOI: http://doi.org/10.4168/aair.2018.10.3.216
Abstract
- PURPOSE
Asthma is a chronic inflammatory airway disease characterized by airway hyperresponsiveness (AHR), inflammation, and remodeling. There is emerging interest in the involvement of the transient receptor potential vanilloid 1 (TRPV1) channel in the pathophysiology of asthma. This study examined whether TRPV1 antagonism alleviates asthma features in a murine model of chronic asthma.
METHODS
BALB/c mice were sensitized to and challenged by ovalbumin to develop chronic asthma. Capsazepine (TRPV1 antagonist) or TRPV1 small interfering RNA (siRNA) was administered in the treatment group to evaluate the effect of TPV1 antagonism on AHR, airway inflammation, and remodeling.
RESULTS
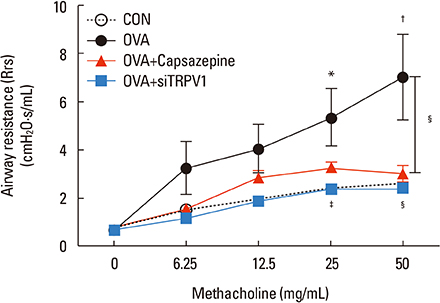
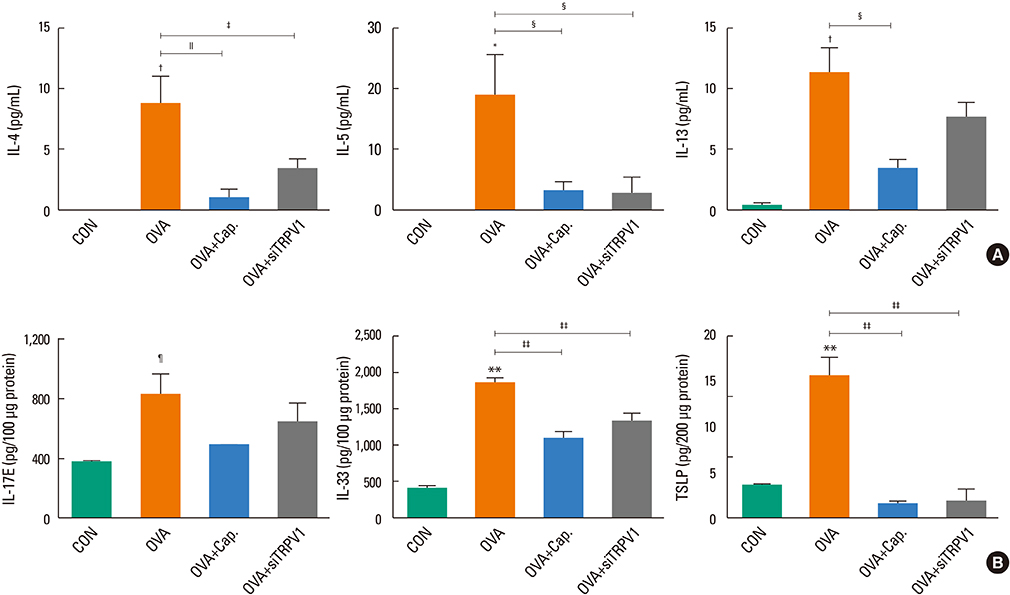
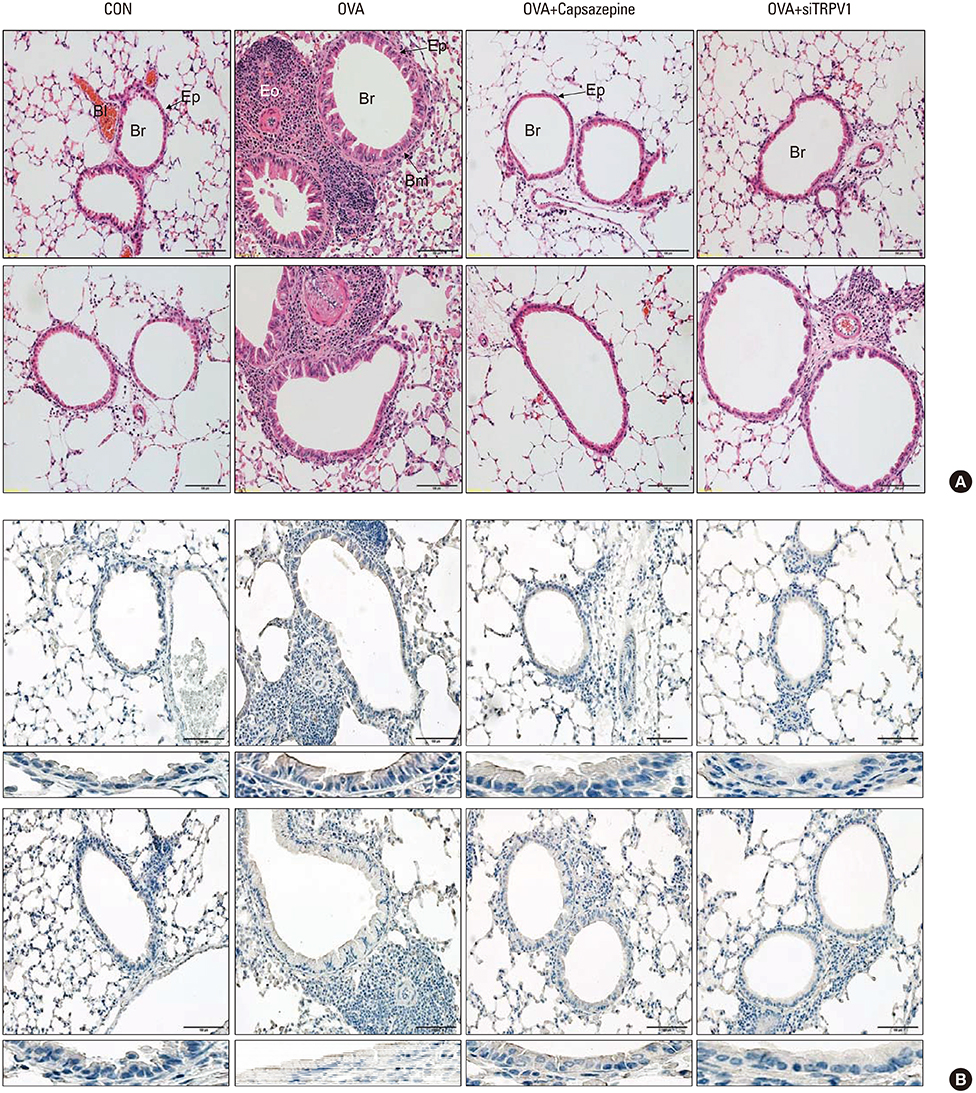
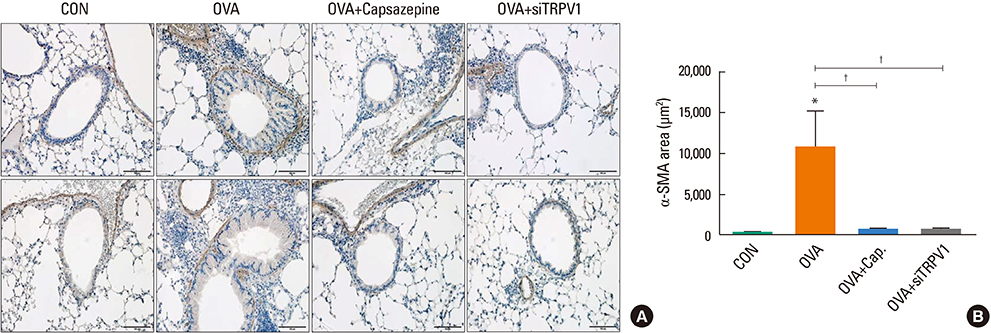
The mice displayed increased AHR, airway inflammation, and remodeling. Treatment with capsazepine or TRPV1 siRNA reduced AHR to methacholine and airway inflammation. Type 2 T helper (Th2) cytokines (interleukin [IL]-4, IL-5, and IL-13) were reduced and epithelial cell-derived cytokines (thymic stromal lymphopoietin [TSLP], IL-33, and IL-25), which regulate Th2 cytokine-associated inflammation, were also reduced. Airway remodeling characterized by goblet cell hyperplasia, increased α-smooth muscle action, and collagen deposition was also alleviated by both treatments.
CONCLUSIONS
Treatment directed at TRPV1 significantly alleviated AHR, airway inflammation, and remodeling in a chronic asthma murine model. The TRPV1 receptor can be a potential drug target for chronic bronchial asthma.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Emerging Role of TRPV1 in Airway Inflammation
Joo-Hee Kim
Allergy Asthma Immunol Res. 2018;10(3):187-188. doi: 10.4168/aair.2018.10.3.187.
Reference
-
1. Rosano A, Loha CA, Falvo R, van der Zee J, Ricciardi W, Guasticchi G, et al. The relationship between avoidable hospitalization and accessibility to primary care: a systematic review. Eur J Public Health. 2013; 23:356–360.
Article2. Bedouch P, Sadatsafavi M, Marra CA, FitzGerald JM, Lynd LD. Trends in asthma-related direct medical costs from 2002 to 2007 in British Columbia, Canada: a population based-cohort study. PLoS One. 2012; 7:e50949.
Article3. Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012. p. 1–8.4. Fajt ML, Wenzel SE. Development of new therapies for severe asthma. Allergy Asthma Immunol Res. 2017; 9:3–14.
Article5. Yoo KH, Ahn HR, Park JK, Kim JW, Nam GH, Hong SK, et al. Burden of respiratory disease in Korea: an observational study on allergic rhinitis, asthma, COPD, and rhinosinusitis. Allergy Asthma Immunol Res. 2016; 8:527–534.
Article6. Zholos AV. TRP channels in respiratory pathophysiology: the role of oxidative, chemical irritant and temperature stimuli. Curr Neuropharmacol. 2015; 13:279–291.
Article7. Adcock JJ. TRPV1 receptors in sensitisation of cough and pain reflexes. Pulm Pharmacol Ther. 2009; 22:65–70.
Article8. Grace MS, Baxter M, Dubuis E, Birrell MA, Belvisi MG. Transient receptor potential (TRP) channels in the airway: role in airway disease. Br J Pharmacol. 2014; 171:2593–2607.
Article9. Clapham DE. TRP channels as cellular sensors. Nature. 2003; 426:517–524.
Article10. Groneberg DA, Niimi A, Dinh QT, Cosio B, Hew M, Fischer A, et al. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med. 2004; 170:1276–1280.
Article11. Mitchell JE, Campbell AP, New NE, Sadofsky LR, Kastelik JA, Mulrennan SA, et al. Expression and characterization of the intracellular vanilloid receptor (TRPV1) in bronchi from patients with chronic cough. Exp Lung Res. 2005; 31:295–306.
Article12. McLeod RL, Correll CC, Jia Y, Anthes JC. TRPV1 antagonists as potential antitussive agents. Lung. 2008; 186:Suppl 1. S59–S65.
Article13. Yang J, Yu HM, Zhou XD, Kolosov VP, Perelman JM. Study on TRPV1-mediated mechanism for the hypersecretion of mucus in respiratory inflammation. Mol Immunol. 2013; 53:161–171.
Article14. McGarvey LP, Butler CA, Stokesberry S, Polley L, McQuaid S, Abdullah H, et al. Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. J Allergy Clin Immunol. 2014; 133:704–712.e4.
Article15. Rehman R, Bhat YA, Panda L, Mabalirajan U. TRPV1 inhibition attenuates IL-13 mediated asthma features in mice by reducing airway epithelial injury. Int Immunopharmacol. 2013; 15:597–605.
Article16. Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D'Amours M, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci U S A. 2009; 106:9099–9104.
Article17. Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med. 1999; 190:895–902.
Article18. Lee HY, Kim IK, Yoon HK, Kwon SS, Rhee CK, Lee SY. Inhibitory effects of resveratrol on airway remodeling by transforming growth factor-β/Smad signaling pathway in chronic asthma model. Allergy Asthma Immunol Res. 2017; 9:25–34.
Article19. Tarkowski M, Vanoirbeek JA, Vanhooren HM, De Vooght V, Mercier CM, Ceuppens J, et al. Immunological determinants of ventilatory changes induced in mice by dermal sensitization and respiratory challenge with toluene diisocyanate. Am J Physiol Lung Cell Mol Physiol. 2007; 292:L207–L214.
Article20. Padrid P, Snook S, Finucane T, Shiue P, Cozzi P, Solway J, et al. Persistent airway hyperresponsiveness and histologic alterations after chronic antigen challenge in cats. Am J Respir Crit Care Med. 1995; 151:184–193.
Article21. von Garnier C, Filgueira L, Wikstrom M, Smith M, Thomas JA, Strickland DH, et al. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J Immunol. 2005; 175:1609–1618.
Article22. Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008; 38:872–897.
Article23. Lambrecht BN, Hammad H. Allergens and the airway epithelium response: gateway to allergic sensitization. J Allergy Clin Immunol. 2014; 134:499–507.
Article24. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997; 389:816–824.
Article25. Baker K, Raemdonck K, Dekkak B, Snelgrove RJ, Ford J, Shala F, et al. Role of the ion channel, transient receptor potential cation channel subfamily V member 1 (TRPV1), in allergic asthma. Respir Res. 2016; 17:67.
Article26. Sadofsky LR, Ramachandran R, Crow C, Cowen M, Compton SJ, Morice AH. Inflammatory stimuli up-regulate transient receptor potential vanilloid-1 expression in human bronchial fibroblasts. Exp Lung Res. 2012; 38:75–81.
Article27. Yu H, Li Q, Zhou X, Kolosov VP, Perelman JM. Transient receptor potential vanilloid 1 receptors mediate acid-induced mucin secretion via Ca2+ influx in human airway epithelial cells. J Biochem Mol Toxicol. 2012; 26:179–186.
Article28. Zhao L, Zhang X, Kuang H, Wu J, Guo Y, Ma L. Effect of TRPV1 channel on the proliferation and apoptosis in asthmatic rat airway smooth muscle cells. Exp Lung Res. 2013; 39:283–294.
Article29. Zhao LM, Kuang HY, Zhang LX, Wu JZ, Chen XL, Zhang XY, et al. Effect of TRPV1 channel on proliferation and apoptosis of airway smooth muscle cells of rats. J Huazhong Univ Sci Technolog Med Sci. 2014; 34:504–509.
Article30. Liu H, Fan X, Wang N, Zhang Y, Yu J. Exacerbating effects of PM2.5 in OVA-sensitized and challenged mice and the expression of TRPA1 and TRPV1 proteins in lungs. J Asthma. 2017; 54:807–817.
Article31. Helyes Z, Elekes K, Németh J, Pozsgai G, Sándor K, Kereskai L, et al. Role of transient receptor potential vanilloid 1 receptors in endotoxin-induced airway inflammation in the mouse. Am J Physiol Lung Cell Mol Physiol. 2007; 292:L1173–L1181.
Article32. Ellis JL, Undem BJ. Inhibition by capsazepine of resiniferatoxin- and capsaicin-induced contractions of guinea pig trachea. J Pharmacol Exp Ther. 1994; 268:85–89.33. Watanabe N, Horie S, Michael GJ, Keir S, Spina D, Page CP, et al. Immunohistochemical co-localization of transient receptor potential vanilloid (TRPV)1 and sensory neuropeptides in the guinea-pig respiratory system. Neuroscience. 2006; 141:1533–1543.
Article34. Raemdonck K, de Alba J, Birrell MA, Grace M, Maher SA, Irvin CG, et al. A role for sensory nerves in the late asthmatic response. Thorax. 2012; 67:19–25.
Article35. Mori T, Saito K, Ohki Y, Arakawa H, Tominaga M, Tokuyama K. Lack of transient receptor potential vanilloid-1 enhances Th2-biased immune response of the airways in mice receiving intranasal, but not intraperitoneal, sensitization. Int Arch Allergy Immunol. 2011; 156:305–312.
Article36. Veronesi B, Oortgiesen M, Roy J, Carter JD, Simon SA, Gavett SH. Vanilloid (capsaicin) receptors influence inflammatory sensitivity in response to particulate matter. Toxicol Appl Pharmacol. 2000; 169:66–76.
Article37. Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008; 226:172–190.
Article38. Lee HY, Rhee CK, Kang JY, Byun JH, Choi JY, Kim SJ, et al. Blockade of IL-33/ST2 ameliorates airway inflammation in a murine model of allergic asthma. Exp Lung Res. 2014; 40:66–76.
Article39. James A, Carroll N. Airway smooth muscle in health and disease; methods of measurement and relation to function. Eur Respir J. 2000; 15:782–789.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of CpG-oligodeoxynucleotides in Chronic Inflammation and Remodeling of Airway in a Murine Model of Bronchial Asthma
- Evolution of Asthma Concept and Effect of Current Asthma Management Guidelines
- Establishment of a murine model based on chronic exposure to domestic house dust mites in Korea
- Effects of Korean Red Ginseng Extracts on Airway Hyperresponsiveness and Inflammation in a Murine Asthma Model
- Effect of DHEA on airway hyperresponsiveness and inflammation in murine model of asthma