J Vet Sci.
2018 Mar;19(2):172-178. 10.4142/jvs.2018.19.2.172.
Korean red ginseng excitation of paraventricular nucleus neurons via non-N-methyl-D-aspartate glutamate receptor activation in mice
- Affiliations
-
- 1Department of Oral Physiology, School of Dentistry and Institute of Oral Bioscience, Chonbuk National University, Jeonju 54896, Korea. skhan@jbnu.ac.kr
- 2Department of Pharmacology, School of Veterinary Medicine, Seoul National University, Seoul 08826, Korea. skhan@jbnu.ac.kr
- 3Daejeon Dong-gu Health Promotion Center, Daejeon 34691, Korea.
- KMID: 2407616
- DOI: http://doi.org/10.4142/jvs.2018.19.2.172
Abstract
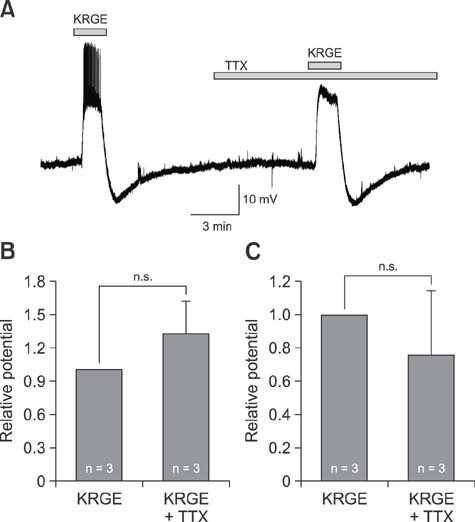
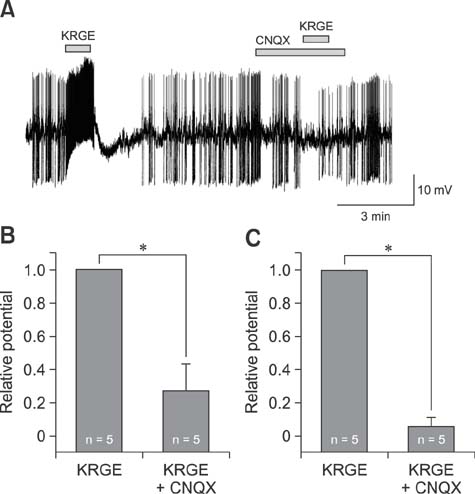
- It has been reported that Korean red ginseng (KRG), a valuable and important traditional medicine, has varied effects on the central nervous system, suggesting its activities are complicated. The paraventricular nucleus (PVN) neurons of the hypothalamus has a critical role in stress responses and hormone secretions. Although the action mechanisms of KRG on various cells and systems have been reported, the direct membrane effects of KRG on PVN neurons have not been fully described. In this study, the direct membrane effects of KRG on PVN neuronal activity were investigated by using a perforated patch-clamp in ICR mice. In gramicidin perforated patch-clamp mode, KRG extract (KRGE) induced repeatable depolarization followed by hyperpolarization of PVN neurons. The KRGE-induced responses were concentration-dependent and persisted in the presence of tetrodotoxin, a voltage sensitive Na+ channel blocker. The KRGE-induced responses were suppressed by 6-cyano-7-nitroquinoxaline-2,3-dione (10 µM), a non-N-methyl-D-aspartate (NMDA) glutamate receptor antagonist, but not by picrotoxin, a type A gamma-aminobutyric acid receptor antagonist. The results indicate that KRG activates non-NMDA glutamate receptors of PVN neurons in mice, suggesting that KRG may be a candidate for use in regulation of stress responses by controlling autonomic nervous system and hormone secretion.
MeSH Terms
-
6-Cyano-7-nitroquinoxaline-2,3-dione
Animals
Autonomic Nervous System
Central Nervous System
Glutamic Acid*
Gramicidin
Hypothalamus
Medicine, Traditional
Membranes
Mice*
Mice, Inbred ICR
Neurons*
Panax*
Paraventricular Hypothalamic Nucleus*
Patch-Clamp Techniques
Picrotoxin
Receptors, GABA
Receptors, Glutamate*
Tetrodotoxin
6-Cyano-7-nitroquinoxaline-2,3-dione
Glutamic Acid
Gramicidin
Picrotoxin
Receptors, GABA
Receptors, Glutamate
Tetrodotoxin
Figure
Reference
-
1. Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999; 58:1685–1693.2. Brunton PJ, Sausbier M, Wietzorrek G, Sausbier U, Knaus HG, Russell JA, Ruth P, Shipston MJ. Hypothalamic-pituitary-adrenal axis hyporesponsiveness to restraint stress in mice deficient for large-conductance calcium- and voltage-activated potassium (BK) channels. Endocrinology. 2007; 148:5496–5506.
Article3. Chen X. Cardiovascular protection by ginsenosides and their nitric oxide releasing action. Clin Exp Pharmacol Physiol. 1996; 23:728–732.
Article4. Cho DH, Bhattarai JP, Han SK. GABAA receptor- and non-NMDA glutamate receptor-mediated actions of Korean red ginseng extract on the gonadotropin releasing hormone neurons. J Ginseng Res. 2012; 36:47–54.
Article5. Choi SH, Lee JH, Pyo MK, Lee BH, Shin TJ, Hwang SH, Kim BR, Lee SM, Oh JW, Kim HC, Bae CS, Rhim H, Nah SY. Mutations Leu427, Asn428, and Leu431 residues within transmembrane domain-I-segment 6 attenuate ginsenoside-mediated L-type Ca2+ channel current inhibitions. Biol Pharm Bull. 2009; 32:1224–1230.
Article6. de Andrade E, de Mesquita AA, Claro Jde A, de Andrade PM, Ortiz V, Paranhos M, Srougi M. Study of the efficacy of Korean Red Ginseng in the treatment of erectile dysfunction. Asian J Androl. 2007; 9:241–244.
Article7. Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004; 25:132–149.
Article8. Feetham CH, Nunn N, Lewis R, Dart C, Barrett-Jolley R. TRPV4 and KCa ion channels functionally couple as osmosensors in the paraventricular nucleus. Br J Pharmacol. 2015; 172:1753–1768.
Article9. Flak JN, Ostrander MM, Tasker JG, Herman JP. Chronic stress-induced neurotransmitter plasticity in the PVN. J Comp Neurol. 2009; 517:156–165.
Article10. Gillis CN. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997; 54:1–8.
Article11. Han SK, Chong W, Li LH, Lee IS, Murase K, Ryu PD. Noradrenaline excites and inhibits GABAergic transmission in parvocellular neurons of rat hypothalamic paraventricular nucleus. J Neurophysiol. 2002; 87:2287–2296.
Article12. Hasegawa H, Suzuki R, Nagaoka T, Tezuka Y, Kadota S, Saiki I. Prevention of growth and metastasis of murine melanoma through enhanced natural-killer cytotoxicity by fatty acid-conjugate of protopanaxatriol. Biol Pharm Bull. 2002; 25:861–866.
Article13. Huang LF, Shi HL, Gao B, Wu H, Yang L, Wu XJ, Wang ZT. Decichine enhances hemostasis of activated platelets via AMPA receptors. Thromb Res. 2014; 133:848–854.
Article14. Ishiuchi S, Tsuzuki K, Yoshida Y, Yamada N, Hagimura N, Okado H, Miwa A, Kurihara H, Nakazato Y, Tamura M, Sasaki T, Ozawa S. Blockage of Ca2+-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat Med. 2002; 8:971–978.
Article15. Jeon BH, Kim CS, Kim HS, Park JB, Nam KY, Chang SJ. Effect of Korean red ginseng on blood pressure and nitric oxide production. Acta Pharmacol Sin. 2000; 21:1095–1100.16. Jin SH, Park JK, Nam KY, Park SN, Jung NP. Korean red ginseng saponins with low ratios of protopanaxadiol and protopanaxatriol saponin improve scopolamine-induced learning disability and spatial working memory in mice. J Ethnopharmacol. 1999; 66:123–129.
Article17. Kaneko H, Nakanishi K. Proof of the mysterious efficacy of ginseng: basic and clinical trials: clinical effects of medical ginseng, Korean red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004; 95:158–162.
Article18. Kim HS, Jung MW, Jang CG, Park WK, Oh KW. Effects of ginseng total saponin on stress-induced analgesia. Korean J Ginseng Sci. 1993; 17:22–28.19. Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods. 1995; 57:27–35.
Article20. Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986; 55:1268–1282.
Article21. Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987; 389:187–203.
Article22. Lee BH, Kim J, Lee RM, Choi SH, Kim HJ, Hwang SH, Lee MK, Bae CS, Kim HC, Rhim H, Lim K, Nah SY. Gintonin enhances performance of mice in rotarod test: involvement of lysophosphatidic acid receptors and catecholamine release. Neurosci Lett. 2016; 612:256–260.
Article23. Lee JH, Jeong SM, Kim JH, Lee BH, Yoon IS, Lee JH, Choi SH, Kim DH, Rhim H, Kim SS, Kim JI, Jang CG, Song JH, Nah SY. Characteristics of ginsenoside Rg3-mediated brain Na+ current inhibition. Mol Pharmacol. 2005; 68:1114–1126.
Article24. Lee JH, Jeong SM, Kim JH, Lee BH, Yoon IS, Lee JH, Choi SH, Lee SM, Park YS, Lee JH, Kim SS, Kim HC, Lee BY, Nah SY. Effects of ginsenosides and their metabolites on voltage-dependent Ca2+ channel subtypes. Mol Cells. 2006; 21:52–62.25. Lee JH, Lee BH, Choi SH, Yoon IS, Shin TJ, Pyo MK, Lee SM, Kim HC, Nah SY. Involvement of batrachotoxin binding sites in ginsenoside-mediated voltage-gated Na+ channel regulation. Brain Res. 2008; 1203:61–67.
Article26. Li DP, Byan HS, Pan HL. Switch to glutamate receptor 2-lacking AMPA receptors increases neuronal excitability in hypothalamus and sympathetic drive in hypertension. J Neurosci. 2012; 32:372–380.
Article27. Mochizuki M, Yoo YC, Matsuzawa K, Sato K, Saiki I, Tono-oka S, Samukawa K, Azuma I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull. 1995; 18:1197–1202.
Article28. Park H, Kim S, Rhee J, Kim HJ, Han JS, Nah SY, Chung C. Synaptic enhancement induced by gintonin via lysophosphatidic acid receptor activation in central synapses. J Neurophysiol. 2015; 113:1493–1500.
Article29. Park JD. Recent studies on the chemical constituents of Korean ginseng (Panax ginseng C. A. Meyer). Korean J Ginseng Sci. 1996; 20:389–396.30. Piet R, Manzoni OJ. Prime time for stress. Nat Neurosci. 2010; 13:1156–1158.
Article31. Renaud LP, Bourque CW. Neurophysiology and neuropharmacology of hypothalamic magnocellular neurons secreting vasopressin and oxytocin. Prog Neurobiol. 1991; 36:131–169.
Article32. Sawchenko PE, Brown ER, Chan RK, Ericsson A, Li HY, Roland BL, Kovács KJ. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Prog Brain Res. 1996; 107:201–222.33. Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res. 2000; 122:61–78.
Article34. Shin TJ, Kim HJ, Kwon BJ, Choi SH, Kim HB, Hwang SH, Lee BH, Lee SM, Zukin RS, Park JH, Kim HC, Rhim H, Lee JH, Nah SY. Gintonin, a ginseng-derived novel ingredient, evokes long-term potentiation through N-methyl-D-aspartic acid receptor activation: involvement of LPA receptors. Mol Cells. 2012; 34:563–572.
Article35. Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987; 385:733–759.
Article36. Sugaya A, Yuzurihara M, Tsuda T, Yasuda K, Kajiwara K, Sugaya E. Proliferative effect of ginseng saponin on neurite extension of primary cultured neurons of the rat cerebral cortex. J Ethnopharmacol. 1988; 22:173–181.
Article37. Sung H, Jung YS, Cho YK. Beneficial effects of a combination of Korean red ginseng and highly active antiretroviral therapy in human immunodeficiency virus type 1-infected patients. Clin Vaccine Immunol. 2009; 16:1127–1131.
Article38. Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983; 6:269–324.
Article39. Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980; 31:410–417.
Article40. Tegnér J, Lansner A, Grillner S. Activity dependent modulation of the burst rate by calcium-dependent potassium channels in lamprey. In : Bower JM, editor. Computational Neuroscience. Boston: Springer;1998. p. 549–554.
Article41. Vuksan V, Sung MK, Sievenpiper JL, Stavro PM, Jenkins AL, Di Buono M, Lee KS, Leiter LA, Nam KY, Arnason JT, Choi M, Naeem A. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis. 2008; 18:46–56.
Article42. Wang ZJ, Sun L, Peng W, Ma S, Zhu C, Fu F, Heinbockel T. Ginseng derivative ocotillol enhances neuronal activity through increased glutamate release: a possible mechanism underlying increased spontaneous locomotor activity of mice. Neuroscience. 2011; 195:1–8.
Article43. Yin H, Park SA, Park SJ, Han SK. Korean red ginseng extract activates non-NMDA glutamate and GABAA receptors on the substantia gelatinosa neurons of the trigeminal subnucleus caudalis in mice. J Ginseng Res. 2011; 35:219–225.
Article44. Yun TK. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. 2001; 16:Suppl. S3–S5.45. Yun TK. Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutat Res. 2003; 523-524:63–74.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Resveratrol attenuates N-methyl-D-aspartate receptor-mediated activation in substantia gelatinosa neurons of trigeminal subnucleus caudalis in mice
- Effect of Chronic Alcohol Intake on Vasopressin and Oxytocin-containing Neurons in the Paraventricular and Supraoptic Nucleus of the Rat Hypothalamus
- Effect of Brain-derived Neurotrophic Factor on Excitatory Synaptic Transmission in Hippocampal Neurons
- Dopaminergic Neurons in the Diencephalon of Striped Field Mouse[Apodemus agrarius coreae]
- The Effects of N-Methyl_D-Aspartic Acid, and Antagonism by Kynurenic Acid on Neurons in the Cathish Retina