J Breast Cancer.
2015 Sep;18(3):225-234. 10.4048/jbc.2015.18.3.225.
Cellular Effect of Curcumin and Citral Combination on Breast Cancer Cells: Induction of Apoptosis and Cell Cycle Arrest
- Affiliations
-
- 1Biochemistry Division, B. R. Doshi School of Biosciences, Sardar Patel University, Vallabh Vidyanagar, India. patel_pinaki@yahoo.com
- 2P. D. Patel Institute of Applied Science, Charotar University of Science and Technology (CHARUSAT), Changa, India.
- KMID: 2407569
- DOI: http://doi.org/10.4048/jbc.2015.18.3.225
Abstract
- PURPOSE
The unmanageable side effects caused by current chemotherapy regimens to treat cancer are an unresolved problem. Although many phytonutrients are useful as chemoprevention without side effects, their effects are slower and smaller than conventional chemotherapy. In the present work, we examined the cumulative effect of two phytonutrients, curcumin and citral, on breast cancer cell lines and compared their effect with the known chemotherapy regimen of cyclophosphamide, methotrexate, and 5-fluorouracil.
METHODS
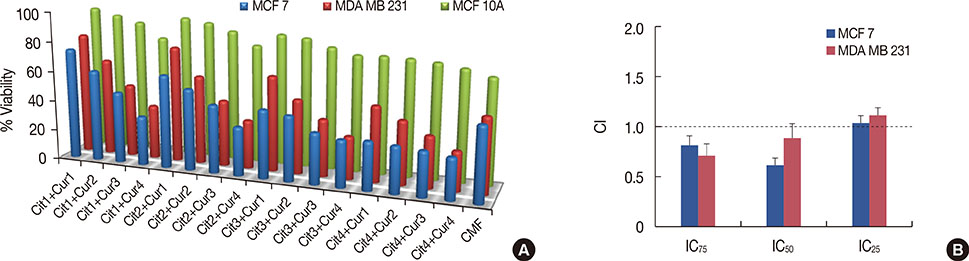
Using cultured breast cancer and normal epithelial cells, the cytotoxic and apoptotic effect of curcumin and citral was evaluated in vitro. The synergistic effect of curcumin and citral was calculated by a combination index study using the method by Chou and Talalay. Cell death pathways and mechanisms were analyzed by measuring intracellular reactive oxygen species (ROS) and apoptotic protein levels.
RESULTS
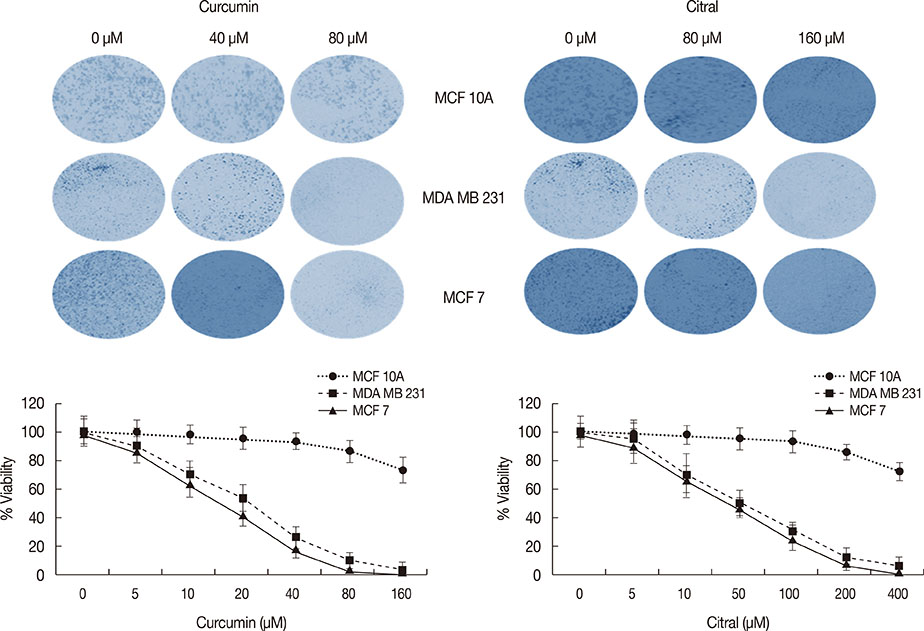
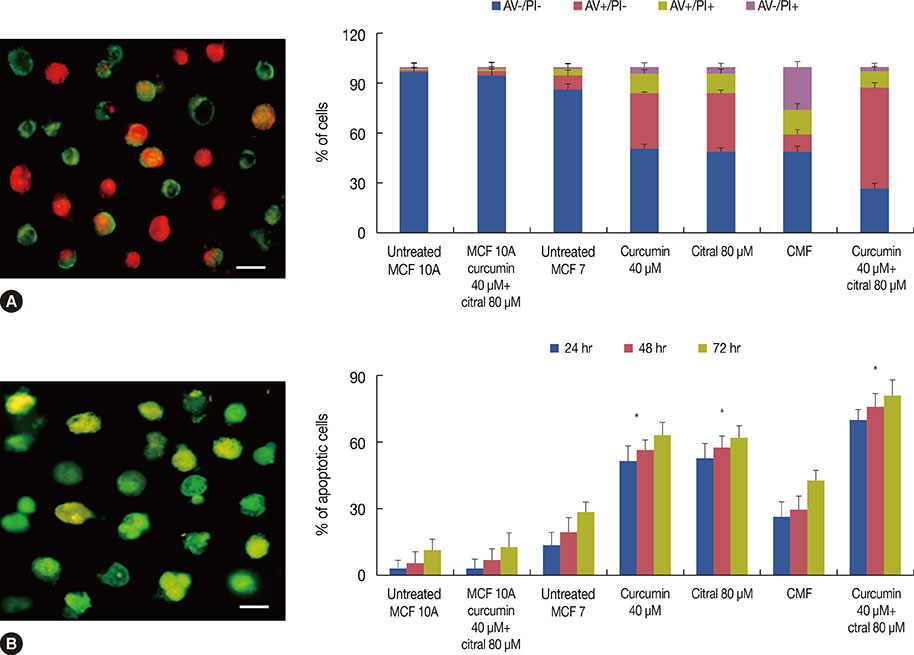
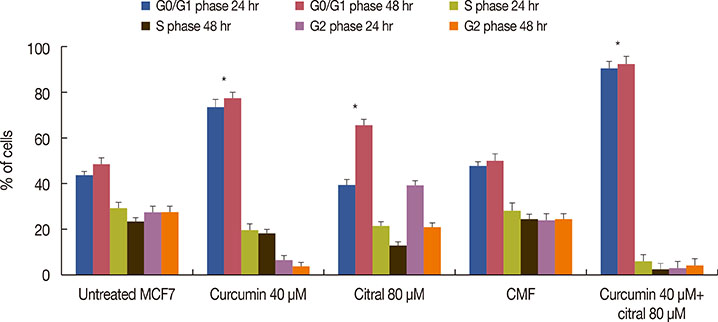
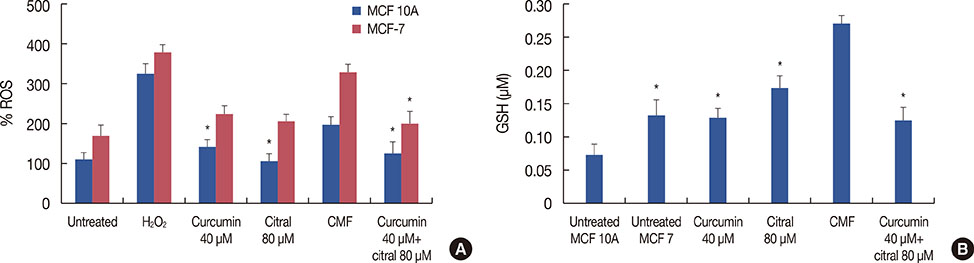
Curcumin and citral caused dose and time dependent cell death and showed a synergistic effect at effective concentration EC50 and above concentrations in breast cancer cells without disturbing normal breast epithelial cells. With combination curcumin and citral treatment, apoptosis induction and cell cycle arrest at G0/G1 phase in breast cancer cells were observed. Curcumin and citral generated ROS and activated p53 and poly (ADP-ribose) polymerase-1 mediated apoptotic pathways.
CONCLUSION
The results of this study suggest that curcumin and citral in combination may be a useful therapeutic intervention for breast cancer.
MeSH Terms
Figure
Reference
-
1. Pan MH, Ho CT. Chemopreventive effects of natural dietary compounds on cancer development. Chem Soc Rev. 2008; 37:2558–2574.
Article2. Lee SJ, Krauthauser C, Maduskuie V, Fawcett PT, Olson JM, Rajasekaran SA. Curcumin-induced HDAC inhibition and attenuation of medulloblastoma growth in vitro and in vivo. BMC Cancer. 2011; 11:144.
Article3. Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009; 11:495–510.
Article4. Ramachandran C, You W. Differential sensitivity of human mammary epithelial and breast carcinoma cell lines to curcumin. Breast Cancer Res Treat. 1999; 54:269–278.
Article5. Shah G, Shri R, Panchal V, Sharma N, Singh B, Mann AS. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass). J Adv Pharm Technol Res. 2011; 2:3–8.
Article6. Rabbani SI, Devi K, Shivananda TN. Studies on antimutagenic effects of citral in mice. J Food Agric Environ. 2004; 2:62–64.7. Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci. 2007; 96:2181–2196.
Article8. Lau AT, Wang Y, Chiu JF. Reactive oxygen species: current knowledge and applications in cancer research and therapeutic. J Cell Biochem. 2008; 104:657–667.
Article9. Bhaumik S, Anjum R, Rangaraj N, Pardhasaradhi BV, Khar A. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett. 1999; 456:311–314.
Article10. Somasundaram S, Edmund NA, Moore DT, Small GW, Shi YY, Orlowski RZ. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002; 62:3868–3875.11. Dudai N, Weinstein Y, Krup M, Rabinski T, Ofir R. Citral is a new inducer of caspase-3 in tumor cell lines. Planta Med. 2005; 71:484–488.
Article12. Xia H, Liang W, Song Q, Chen X, Chen X, Hong J. The in vitro study of apoptosis in NB4 cell induced by citral. Cytotechnology. 2013; 65:49–57.
Article13. Zhang T, Zhang Q, Chen D, Jiang J, Zhou Q. Growth inhibition of human breast cancer cell line MDA-MB-231 by rosiglitazone through activation of PPARgamma. Chin J Clin Oncol. 2008; 5:407–412.
Article14. Reynolds CP, Maurer BJ. Evaluating response to antineoplastic drug combinations in tissue culture models. Methods Mol Med. 2005; 110:173–183.
Article15. Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, et al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000; 35:206–221.
Article16. Sies H, Akerboom TP. Glutathione disulfide (GSSG) efflux from cells and tissues. Methods Enzymol. 1984; 105:445–451.17. Singh RP, Dhanalakshmi S, Agarwal R. Phytochemicals as cell cycle modulators: a less toxic approach in halting human cancers. Cell Cycle. 2002; 1:156–161.
Article18. Chaouki W, Leger DY, Liagre B, Beneytout JL, Hmamouchi M. Citral inhibits cell proliferation and induces apoptosis and cell cycle arrest in MCF-7 cells. Fundam Clin Pharmacol. 2009; 23:549–556.
Article19. Shen L, Ji HF. Theoretical study on physicochemical properties of curcumin. Spectrochim Acta A Mol Biomol Spectrosc. 2007; 67:619–623.
Article20. Liu Q, Loo WT, Sze SC, Tong Y. Curcumin inhibits cell proliferation of MDA-MB-231 and BT-483 breast cancer cells mediated by down-regulation of NFkappaB, cyclinD and MMP-1 transcription. Phytomedicine. 2009; 16:916–922.
Article21. Syng-Ai C, Kumari AL, Khar A. Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2. Mol Cancer Ther. 2004; 3:1101–1108.22. Fang HY, Chen SB, Guo DJ, Pan SY, Yu ZL. Proteomic identification of differentially expressed proteins in curcumin-treated MCF-7 cells. Phytomedicine. 2011; 18:697–703.
Article23. Shyur LF, Lee SH, Chang ST, Lo CP, Kuo YH, Wang SY. Taiwanin A inhibits MCF-7 cancer cell activity through induction of oxidative stress, upregulation of DNA damage checkpoint kinases, and activation of p53 and FasL/Fas signaling pathways. Phytomedicine. 2010; 18:16–24.
Article24. Ataee R, Ataie A, Shadifar M, Nasri N, Hagghi H, Hayati E. Synergic effect of curcumin and melatonin on proliferation and apoptosis of HT29 colorectal cancer cell line. Res Pharm Sci. 2012; 7:S117.25. Li H, Jin L, Wu F, Li X, You J, Cao Z, et al. Effect of curcumin on proliferation, cell cycle, and caspases and MCF-7 cells. Afr J Pharm Pharmacol. 2012; 6:864–870.26. Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995; 358:1–3.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells
- Curcumin Induces Apoptosis and Inhibits Metalloproteinase Activity in Renal Cancer Cell Line
- Mechanism Underlying Curcumin-induced Apoptosis and Cell Cycle Arrest on SCC25 Human Tongue Squamous Cell Carcinoma Cell Line
- Growth inhibition by fusidic acid in cervical, thyroid, and breast carcinoma cell lines
- Kaempferol Synergistically Enhances Cisplatin-induced Apoptosis and Cell Cycle Arrest in Colon Cancer Cells